Antigens, which are foreign substances, trigger the production of antibodies by the immune system. These antibodies are specific to an antigen or part of it and can destroy or neutralize cells and substances through various mechanisms. Antibodies have therapeutic and diagnostic applications in human health, making them a subject of extensive research. The first monoclonal antibodies, of murine origin, were developed over 35 years ago but resulted in unwanted human anti-murine antibody responses in patients. To reduce or eliminate these side effects, modifications were made, resulting in the development of chimeric, humanized, and human versions of monoclonal antibodies. Innovative modifications were also made to the size and biological activities of antibodies for in vivo diagnostic and therapeutic applications.
Biotechnology has revolutionized the production of monoclonal antibodies, making their development and production more cost-effective and scalable. Recombinant DNA technology enables the production of monoclonal antibodies in large quantities, and humanization techniques can minimize the risk of immune reactions in patients. Monoclonal antibodies have a wide range of applications in medicine, including cancer treatment, autoimmune disorders, and infectious diseases. They are also extensively used in research, but the cost of their production can limit their accessibility to some research groups. Overall, biotechnology has made monoclonal antibodies a valuable tool for medical research and therapy.
Advantages of Monoclonal Antibodies in Biotechnology
Specificity
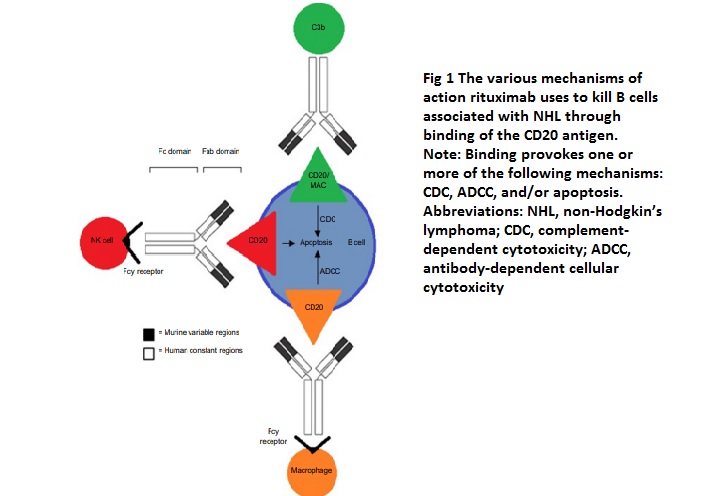
Monoclonal antibodies possess high specificity for the antigen they are designed to target, allowing them to differentiate between various cell types, molecules, or even specific amino acids. Rituximab works by binding to the CD20 antigen through its Fab domain and using its Fc domain to recruit immune effector functions that lead to the destruction of B-cells. The exact mechanisms through which rituximab achieves B-cell depletion in vivo are not fully understood, but evidence suggests that it stabilizes CD20 on lipid rafts, which promotes ADCC (antibody-dependent cell-mediated cytotoxicity). This process is mediated by Fcγ receptors found on the surface of granulocytes, macrophages, and NK cells. Other possible mechanisms of action include complement-dependent cytotoxicity activated by C1q binding and the subsequent lytic cascade, as well as apoptosis.
Reproducibility
Monoclonal antibodies can be produced in large quantities and with consistent quality, ensuring the reproducibility of experiments and therapies. Monoclonal antibodies are targeted therapies used for neoplastic conditions, autoimmune diseases, post-transplant immunosuppressants, and infectious diseases. They offer high affinity and specificity but can develop resistance. Combination monoclonal antibody cocktails are being developed to prevent neutralization escape. Currently, there are 70 monoclonal antibodies in development or clinical trials to treat COVID-19, with FDA emergency use authorization granted for five agents.
Large-scale production
Recombinant DNA technology enables the production of monoclonal antibodies in large quantities, making the production process more economically feasible and capable of being scaled up. The prevention of HIV infection in susceptible women would require more than 3 tonnes of mAbs per year per million women. The current manufacturing processes based on mammalian cell cultures in stirred tank bioreactors would not be able to meet such high demands, even with the largest available fermenter volumes and optimal conditions. Additionally, such very-large-scale reactor setups would require expensive stainless steel equipment and lack the benefits of single-use technologies that have become popular in the pharmaceutical industry. Thus, a new era of very-large-scale production, similar to the food processing industry, would be needed to meet the high demand for mAbs.
Long shelf-life
Monoclonal antibodies are highly stable and can retain their efficacy and potency even after being stored for extended periods of time. A crucial aspect of developing monoclonal antibodies is predicting their long-term stability. However, current methods of accelerated stability studies lack robustness. A new study has used accelerated stability studies and a first-order degradation kinetic model to predict the long-term stability of multiple monoclonal antibody formulations. The study can predict the stability of a protein at its intended storage condition (5°C) using up to six months of data from different temperatures. The model was validated by comparing the 95% prediction interval and experimental stability data from up to 36 months. Compared to current methods, the model demonstrated improved accuracy, speed, and robustness in predicting long-term stability, which could aid the refinement of regulatory guidelines for monoclonal antibodies. Extrapolation for biologics could be allowed during developmental, clinical, and marketing authorization applications, similar to established protocols for small molecules.
Humanization
Humanization of monoclonal antibodies is advantageous as it minimizes the chances of provoking an immune reaction in patients. This is achieved by modifying the non-human antibody sequence to make it more similar to a human antibody. As a result, the therapeutic antibody is less likely to be recognized as foreign by the human immune system. This increases the safety and efficacy of the monoclonal antibody as a therapeutic agent. In addition to reducing the likelihood of triggering an immune response, humanization can also increase the half-life of the monoclonal antibody in the body. This allows for longer-lasting therapeutic effects and reduces the need for frequent dosing. Humanisation has become a standard practice in the development of monoclonal antibodies and has contributed to the success of many therapeutic antibodies. By creating antibodies that are more similar to human antibodies, humanization has improved the safety, efficacy, and convenience of monoclonal antibody therapy.
Limitations of Monoclonal Antibodies in Biotechnology
High Cost
The development and production of monoclonal antibodies can be expensive, which limits their access to certain patient populations and research groups. Monoclonal antibodies (mAbs) have revolutionized the treatment of various diseases, including cancer, autoimmune disorders, and infectious diseases. However, the cost of these treatments can be prohibitively high, making them inaccessible to some patients. They are also widely used in research, but their production cost can limit their accessibility to smaller research groups with limited budgets.
Immunogenicity
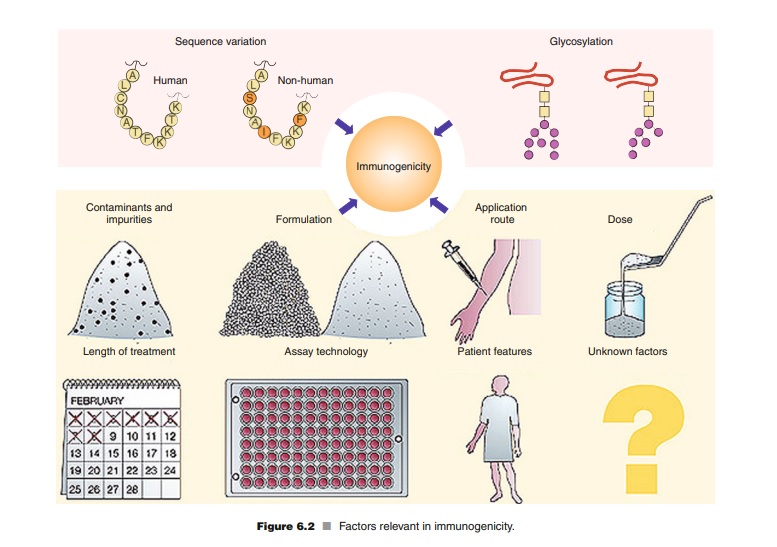
Monoclonal antibodies can induce an immune response in the patient, leading to the formation of antibodies against the therapeutic agent itself. This can reduce the effectiveness of the therapy or cause adverse reactions. Monoclonal antibodies have undergone a paradigm shift in immunogenicity. Early murine monoclonal antibodies triggered a HAMA (Human antibodies to murine antibodies) response, which limited their clinical success. Humanization methods were introduced to reduce immunogenicity, but even fully human monoclonal antibodies can induce antibodies. Aggregates have been identified as a major cause of immunogenicity, and the antibodies’ Fc functions and binding targets also influence their immunogenicity. Chronic treatment and higher doses may be less immunogenic than episodic treatment and lower doses, but the interpretation of these data is difficult. There is little difference in immunogenicity between subcutaneous and intravenous administration of monoclonal antibodies.
Immune reactions to therapeutic proteins can cause various effects such as anaphylaxis, hypersensitivity, skin reactions, and serum sickness. These effects are more common when large amounts of nonhuman proteins are used, but they are relatively rare with modern biotechnology-derived products that are highly purified human proteins administered in low amounts. However, immune response-related side effects are still relatively common when high doses of monoclonal antibodies are used in treatment.
Considerations Before Conducting Clinical Trials With Human mAbs
Before entering a clinical setting with a human mAb, there must be a strong reason supported by preclinical data. The more data available, the better the decision-making to reduce time and expenses. Attempting clinical trials with minimal data can be nonoptimal and cause issues. Patient recruitment is crucial to the success of early clinical trials with human mAbs, and trial design should focus on those patients who are most likely to respond to therapy. The availability of enough antibodies for all patients is also a practical limitation in clinical trials, and sufficient supplies must be available to meet demands after approval.
Challenges in screening human mAbs against tissue samples
To screen human mAbs effectively, a reproducible target is necessary. In some cases, these mAbs are tested against tissue samples. However, the availability of rare tissues can be prohibitive, and long-term cultures may not accurately represent the original cell lines. While genetically engineered targets can be used, tissue extracts or biopsy tissues are often limited in supply for screening purposes.
The Importance of Fc Glycosylation and Glycoengineering for the Efficacy of mAbs
The Fc portion of mAbs is essential in therapeutic applications as it enables a complete and efficient immune response. However, the exact mode of action of mAbs in patients is not always clear and varies. Some mAbs bind to antigens, while others target cell surface receptors or trigger apoptosis. ADCC (Antibody-dependent cell-mediated cytotoxicity) plays a significant role in the in vivo efficacy of mAbs, but glycosylation of the Fc region poses limitations. Glycoengineering of the Fc region has been attempted to make mAbs more human-like, and fucose branching must be removed for optimal integration with the human effectors’ system. The cell line used for antibody production affects the carbohydrate moiety and the in vivo efficacy of mAbs.
Ethical Considerations in the Production and Use of Monoclonal Antibodies for Medical Treatment
The production of monoclonal antibodies often involves the use of animals for immunization and antibody production, which raises ethical concerns about animal welfare and the use of animal-derived products in human therapy. Monoclonal antibodies are created from human cells and tissues, and their use in medical treatment raises ethical concerns about using leftover body material for research purposes. The issue of informed consent and patient autonomy is also raised. Some argue that the principle of solidarity, which emphasizes using resources for the greater good, should take priority over patient autonomy.
References
- F. Buyel, R.M. Twyman, R. Fischer, “Very-large-scale production of antibodies in plants: The biologization of manufacturing, Biotechnology Advances, Volume 35, Issue 4, 2017, Pages 458-465, ISSN 0734-9750, https://doi.org/10.1016/j.biotechadv.2017.03.011.
- Demidem A, Lam T, Alas S, Hariharan K, Hanna N, Bonavida B, “Chimeric anti-CD20 (IDEC-C2B8) monoclonal antibody sensitizes a B cell lymphoma cell line to cell killing by cytotoxic drugs”, Cancer Biother Radiopharm. 1997; 12(3):177–186.
- Schellekens H (2002), “Bioequivalence and the immunogenicity of biopharmaceuticals”, Nat Rev Drug Discov 1:1–7.
- Borrebaeck C, Carlsson R (2001) Human therapeutic antibodies. Curr Opin Pharmacol 1:404–408.
- Chames P, Regenmortel MV, Weiss E, Baty aspartic acid (2009) Therapeutic antibodies: successes, limitations, and hopes for the future. Br J Pharmacol 157:220–233.
- Evans HM (2002), “What’s wrong with “retained organs”? Some personal reflections in the afterglow of “Alder Hey”, J Clin Pathol 54:824–826.



