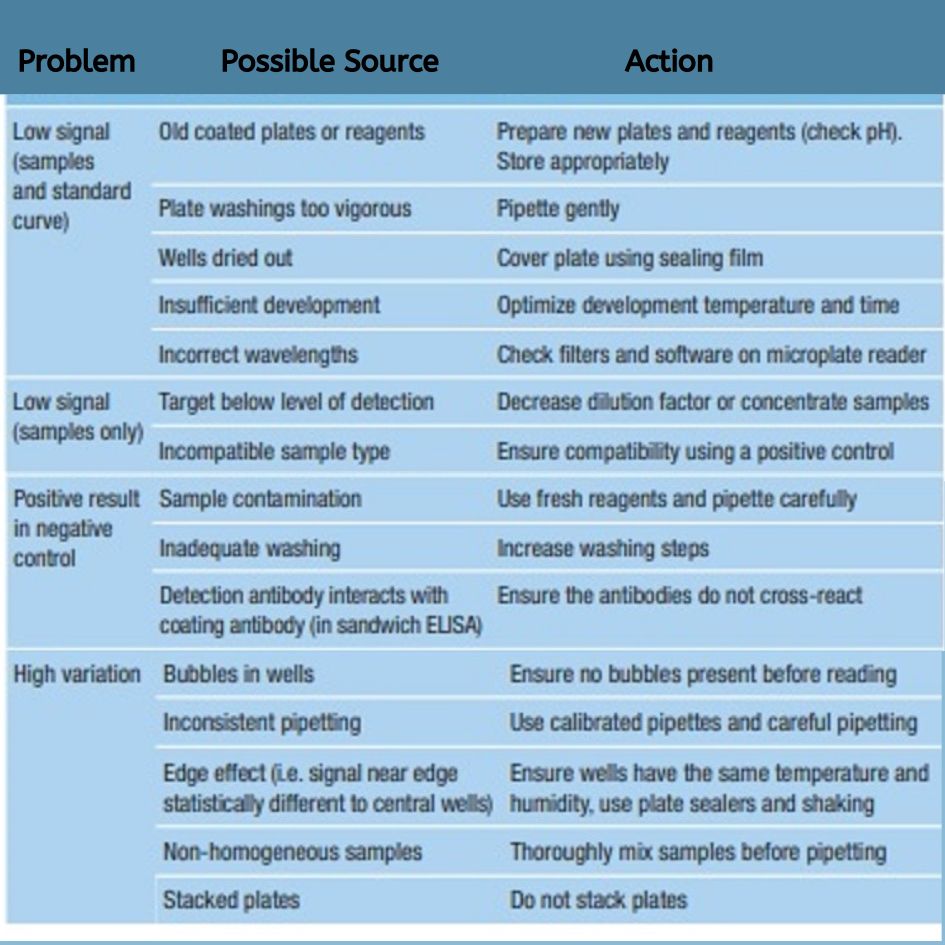
ELISA assays, while widely used and effective, face several challenges that can impact their accuracy and reliability. Key issues include cross-reactivity, where non-target proteins bind to antibodies, leading to false positive or negative results. Variability in sample preparation, including inconsistencies in pipetting and washing steps, can affect reproducibility and precision. Interference from background signal or molecules, and variability in reagents or protocols, can also skew results. Standardization of techniques and equipment, such as plate reader and plate washer, is crucial to minimize these issues. Additionally, the accuracy of ELISA can be limited by factors like improper dilution, affinity of antibody pairs, and inconsistencies in room temperature or wavelength settings. Ensuring proper controls, optimizing wash buffer, and carefully following procedures are essential for achieving reliable measurements and overcoming these challenges.
Troubleshooting ELISA Assay Challenges
Improving ELISA Components
|
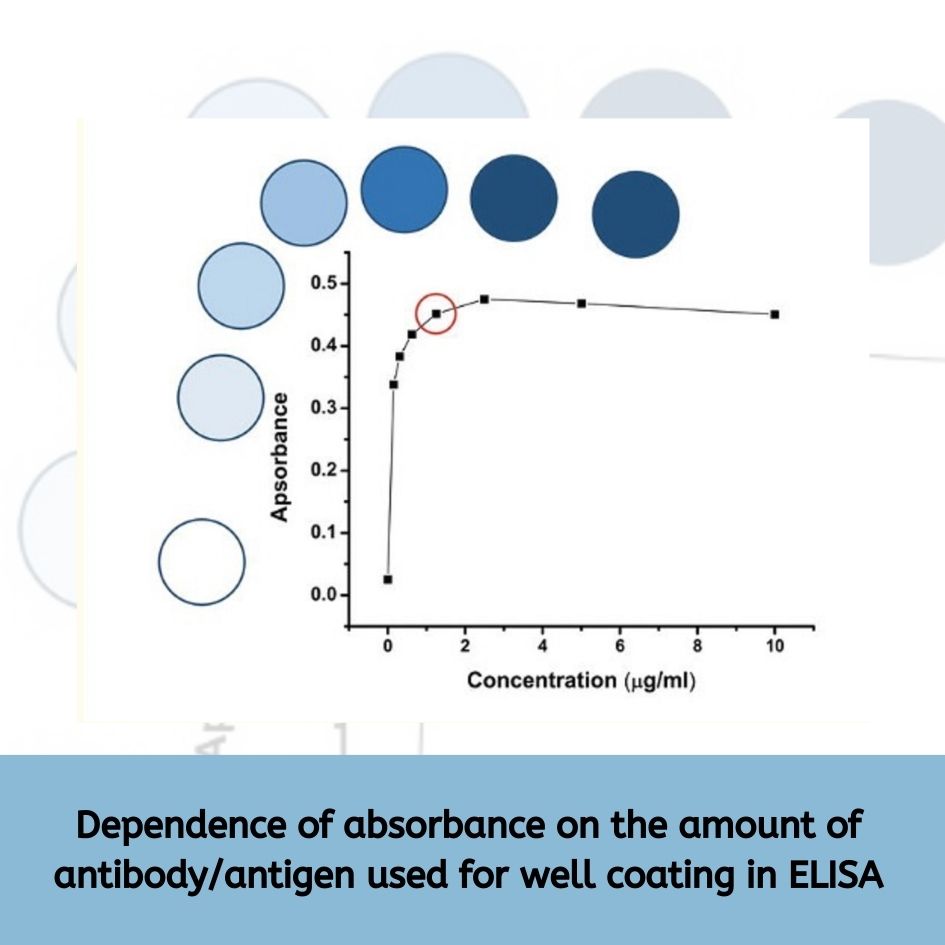
Capture Antibody Concentration |
Test different concentrations in the coating buffer for strong antigen binding without unwanted interactions. |
|
Blocking Buffer |
Try different solutions and concentrations to reduce background noise and improve signal clarity. |
|
Standard Diluent |
Match it closely to the sample’s matrix; use spike-and-recovery tests if exact matching isn’t possible. |
|
Sample Concentration |
Test various sample concentrations. Avoid signals that are too strong or too weak; validate with spike-and-recovery experiments. |
|
Detection Antibody Concentration |
Adjust the detection antibody concentrations, maximize sensitivity and minimize non-specific binding. |
|
Enzyme Conjugate Concentration |
Vary concentrations as per kit guidelines. Optimize signal strength and maintain linearity. |
|
Signal Detection Substrate |
Choose the substrate based on the needed sensitivity and antigen concentration. Ensure it detects the target well without causing background issues. |
Applications on Optimization of Elisa Assay
- The Taguchi method improved ELISA by changing antigen levels to make the best standard curve. It looked at how different factors interact to improve absorbance predictions and found key elements that cause background noise. This method made optimization easier and made ELISA more reliable and accurate.
- New ELISA tests for VL (Visceral Leishmaniasis) use novel antigens like Ld-ESM. These advancements make the tests more specific and sensitive, improving their accuracy in field diagnostics.
- An optimized ELISA for SARS-CoV-2 antibodies was designed for Sub-Saharan Africa, focusing on detecting antibodies in asymptomatic and mildly symptomatic cases. ROC analysis helped set cutoff values to improve diagnostic accuracy, despite some challenges with N-IgM sensitivity.
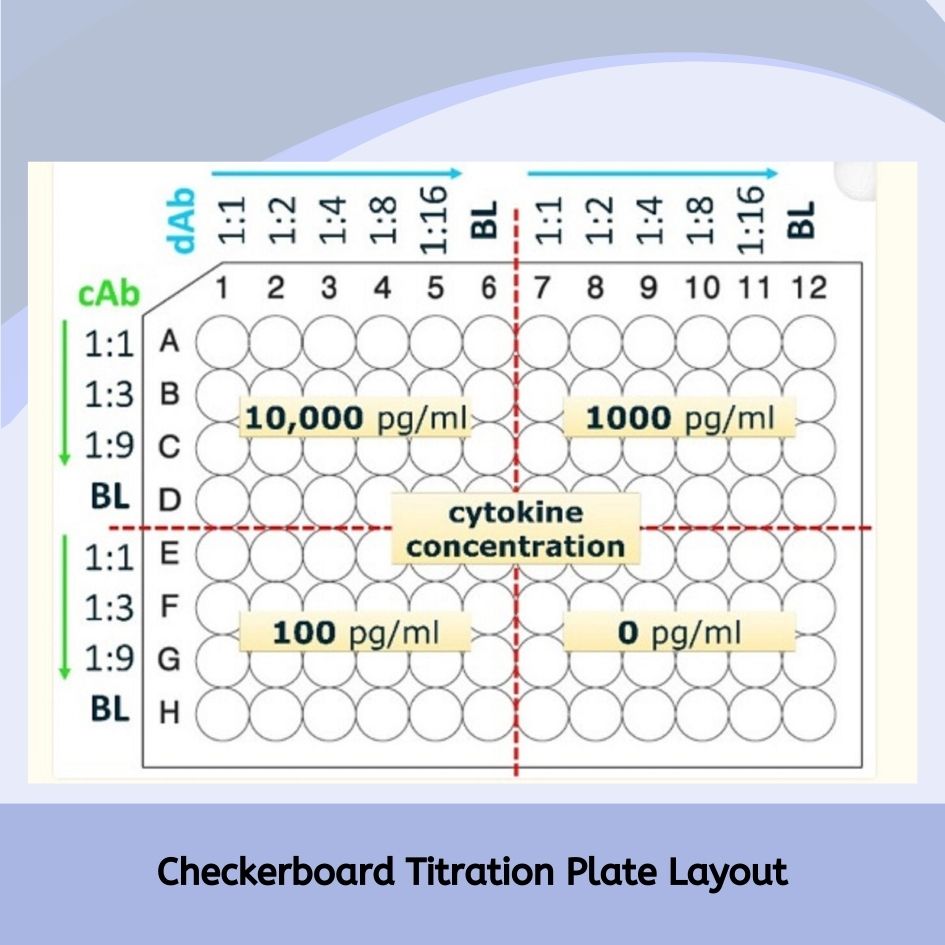
- Each part of the ELISA assay, like antibodies, blocking agents, buffers, enzyme conjugates, and substrates, is optimized to improve the new ELISA protocol. Concentrations and times for coating, blocking, sample incubation, and detection are adjusted to find the best conditions. Often, a checkerboard titration grid method is used to optimize two components at the same time.
- Optimized capture ELISAs for chicken cytokines are very sensitive, showing minimal variation between plates for dependable detection of native cytokines. Including a suitable matrix in the calibration curve is important for determining absolute concentrations. These ELISAs are cost-effective and easy to make, making them widely useful in poultry immunology research.
Explore Our Full Range of ELISA Kits
Whether you’re testing human, animal, or plant samples, MyBioSource offers over 1 million ELISA kits covering thousands of analytes across every major species.
References
- Shah, K., & Maghsoudlou, P. (2016). Enzyme-linked immunosorbent assay (ELISA): the basics. British journal of hospital medicine, 77(7), C98-C101
- Taguchi optimisation of ELISA procedures ) Csaba Jeney , Orsolya Dobay, Anna Lengyel, Eva Adam, Istvan Nasz ´ ´ ´ ´´ Institute of Microbiology, Semmelweis Medical School, NagyÕarad ter 4. Budapest, H-1089 Hungary ´ ´ Microbiological-Virological Research Group of the Hungarian Academy of Sciences, Budapest, Hungary Received 17 February 1998; revised 30 June 1998; accepted 7 September 1998
- Rajasekariah, G. H. R., Ryan, J. R., Hillier, S. R., Lisa, P. Y., Stiteler, J. M., Cui, L., … & Martin, S. K. (2001). Optimisation of an ELISA for the serodiagnosis of visceral leishmaniasis using in vitro derived promastigote antigens. Journal of immunological methods, 252(1-2), 105-119.
- Oluka, G. K., Namubiru, P., Kato, L., Ankunda, V., Gombe, B., Cotten, M., … & Serwanga, J. (2023). Optimisation and Validation of a conventional ELISA and cut-offs for detecting and quantifying anti-SARS-CoV-2 Spike, RBD, and Nucleoprotein IgG, IgM, and IgA antibodies in Uganda. Frontiers in immunology, 14, 1113194.
- Krzysica P, Verhoog L, de Vries S, Smits C, Savelkoul HFJ, Tijhaar E. Optimization of Capture ELISAs for Chicken Cytokines Using Commercially Available Antibodies. Animals (Basel). 2022 Nov 4;12(21):3040. doi: 10.3390/ani12213040. PMID: 36359163; PMCID: PMC9658146.
- Parandakh, A., Ymbern, O., Jogia, W., Renault, J., Ng, A., & Juncker, D. (2023). 3D-printed capillaric ELISA-on-a-chip with aliquoting. Lab on a Chip, 23(6), 1547-1560.
- Hernández, C. A., Pérez-Bernal, M., Abreu, D., Valdivia, O., Delgado, M., Dorta, D., … & Sánchez-Ríos, J. M. (2023). Step-by-step full factorial design to optimize a quantitative sandwich ELISA. Analytical Biochemistry, 674, 115195
- Ambarsari, C., Suryadi, H., & Yanuar, A. Production of Anti-Recombinant Human Insulin Antibody and Validation by Indirect ELISA.




