In the United States, clinical laboratories that perform tests on human specimens to assist in disease diagnosis, prevention, or treatment, or to evaluate an individual’s health status, are subject to regulation by the Clinical Laboratory Improvement Amendments (CLIA). These laboratories must obtain CLIA certification from the Centers for Medicare & Medicaid Services (CMS) by meeting specific quality control standards and criteria. The goal of the CLIA regulations is to ensure that laboratory data is reliable and accurate, that test validation and performance are appropriately documented, and that test results are consistent, regardless of where the test is conducted.
Importance of quality and accuracy in laboratory testing
The final CLIA rule places greater emphasis on quality assessment for preferably analytic and post-analytic processes, while proposing a reduction in quality control (QC) for analytic processes. However, this proposal is not included in the regulations themselves, but rather in the State Operations Manual (SOM), which offers guidelines for implementing the regulations. The SOM suggests that laboratories may be able to reduce QC from 2 levels per day to 2 levels per week or even 2 levels per month for measurement procedures and instruments with built-in controls. These so-called equivalent QC (EQC) procedures would be particularly useful for point-of-care testing applications, where operators may have limited laboratory experience and analytic skills.
Quality standards in clinical and research laboratories
The Centers for Medicare & Medicaid Services (CMS) established quality standards for clinical laboratories to protect patients. These standards require laboratories that report patient-specific test results to meet the CLIA quality standards or an equivalent standard from an approved accrediting organization. These standards help ensure the validity of laboratory test results. Research laboratories have more limited quality-assurance processes in place, which can pose a problem for the return of research results to participants. Concerns exist over the effectiveness of quality procedures in research laboratories in general, due to the variability that exists from laboratory to laboratory. This is because there is no minimum QMS requirement for research laboratories, unlike the requirement for clinical laboratories to meet CLIA standards.
Key elements in Quality assurance and Quality control
The key elements to consider for the development of a research quality management system includes staff proficiency, training, and competency programs, staff compliance documentation, equipment validation and calibration, supplier management, control of bio specimen collection and tracking, documentation of pre-analytical parameters, measurement and analysis of key process indicators, and system security. While not all of these elements may be necessary for every research setting, they represent the minimum considerations for quality assurance and control implementation and auditing.
The recordkeeping and document control aspects of a research quality management system should include a data quality management system for clinical data records, accessibility of policies and procedures, documentation records, external document monitoring, staff training records, and supply records. Internal audits of the program and its policies, both scheduled and unscheduled, should also be conducted. This includes an audit for the accuracy of annotation data and patient data associated with biospecimens, compliance with institution policies, and standard operating procedures (SOPs). Biospecimen resources should ensure that SOPs are written, reviewed, appropriately approved, and regularly reviewed and updated.
Development of a research-grade QMS to mirror the clinical QMS using CLIA
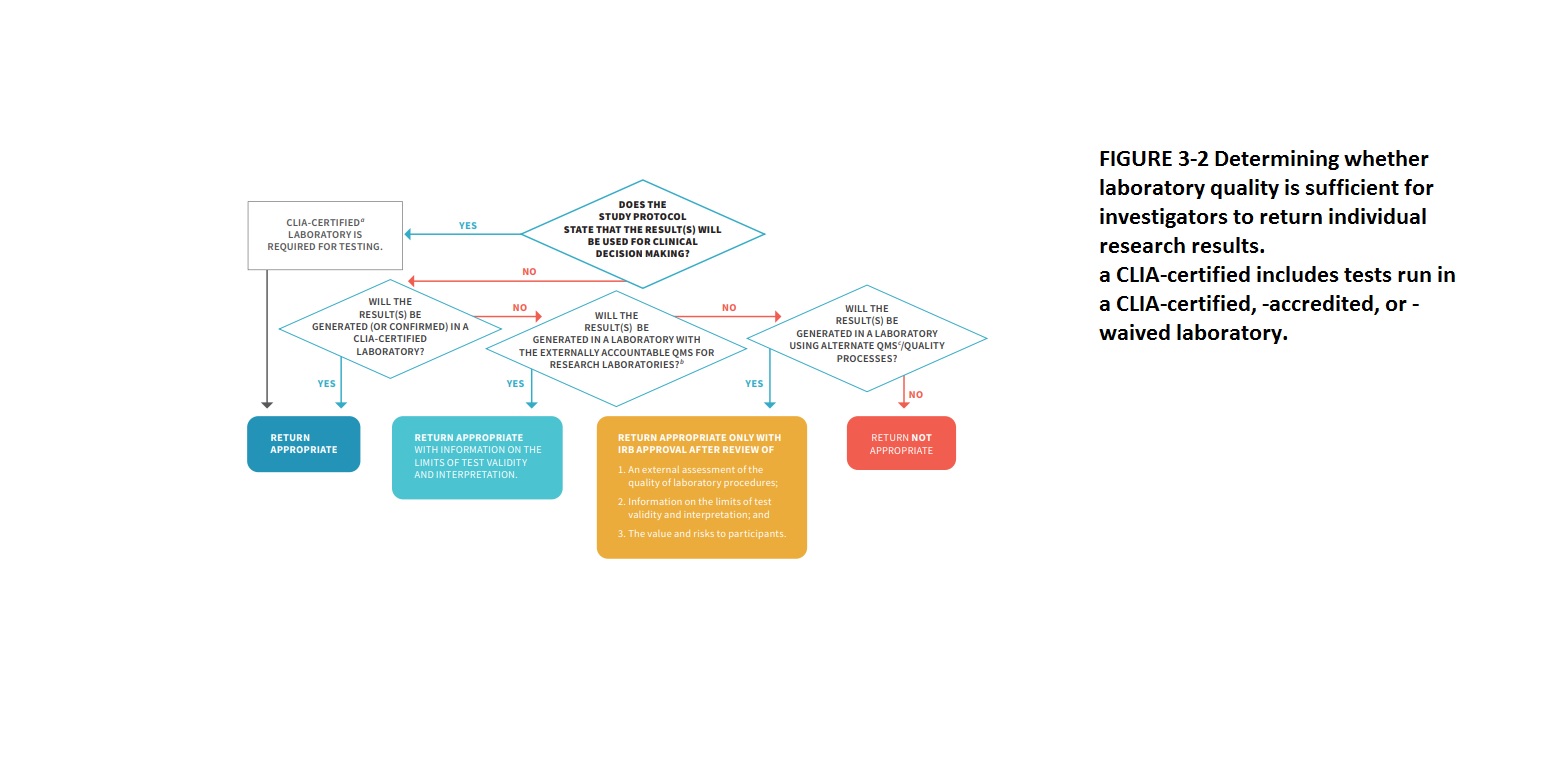
The two important aspects that should be considered when returning individual research results: are (1) whether the results will be used in clinical decision-making in the study protocol, which requires the results to be generated in a CLIA-certified laboratory, and (2) the validity of the results. If the results will not be used in clinical decision-making, other pathways can be used for returning results as long as the limitations of test validity and interpretation are provided. To ensure the quality of research results, investigators should either perform their testing in a CLIA-certified laboratory, use the NIH-led research QMS when available, or use an alternate QMS that is deemed sufficient by an independent review process. The flowchart can also be used to determine whether to return an unanticipated result to a participant.
Steps followed for ensuring quality and accuracy in Laboratory under CLIA regulations
The steps in ensuring quality and accuracy in laboratory testing under CLIA include the following:
Personnel qualifications: laboratories must employ qualified personnel, including licensed medical directors, to oversee testing procedures and ensure accurate and reliable results.
Quality control and quality assurance: laboratories must establish quality control and quality assurance programs to monitor testing procedures and equipment, maintain accurate records, and ensure the reliability and accuracy of test results.
Proficiency testing: Clinical laboratories in the US are required to take part in proficiency testing programs to assess their performance and ensure the accuracy and dependability of their test results when compared to other laboratories.
Test system validation: laboratories must validate all test systems and methods to ensure that they are appropriate for their intended use and produce accurate and reliable results.
Patient test management: laboratories must establish procedures for the proper collection, labeling, and handling of patient specimens to ensure accurate test results and patient safety.
Test result reporting: laboratories must report test results accurately and promptly to the appropriate parties, including the patient’s healthcare provider.
By following these steps, laboratories can ensure the quality and accuracy of their testing procedures and provide reliable results that contribute to the effective diagnosis and treatment of diseases.
Importance of documentation, corrective actions, and monitoring in ensuring the quality and accuracy of test results
Documenting all steps of the laboratory testing process, including the collection, processing, analysis, and reporting of data, is crucial for ensuring the quality and accuracy of test results. Corrective actions must be taken if errors or discrepancies are identified during the testing process or when reviewing results. Monitoring of quality control measures and ongoing training and competency assessment of laboratory personnel is also important to maintain the quality and accuracy of testing. All of these measures help to ensure that the laboratory testing process meets the requirements of CLIA regulations and produces reliable and accurate results that can be used for clinical decision-making.
Regulatory authority responsibilities for inspecting, assessing compliance, and enforcing penalties for non-compliance under CLIA
Under CLIA, regulatory authorities such as the Centers for Medicare & Medicaid Services (CMS) and state survey agencies are responsible for inspecting and assessing compliance of clinical laboratories with CLIA regulations. These regulatory authorities conduct surveys, audits, and inspections of laboratories to ensure they meet the requirements for quality control, quality assurance, proficiency testing, personnel standards, and other CLIA-mandated standards. They also have the authority to enforce penalties, such as fines, suspension, or revocation of laboratory certification, for laboratories found to be in non-compliance with CLIA regulations. The purpose of these regulatory activities is to protect public health by ensuring that laboratory testing is accurate, reliable, and timely.
Although the implementation of a quality management system (QMS) should be tailored to fit the unique needs of each research setting, there are some common requirements for establishing such systems in university laboratories, academic medical centers, and research and development laboratories in the industry. These include a commitment to and investment in the QMS by investigators, support from staff and faculty at all levels, thorough training in quality practices, and the support of general management, the department, or the institution.
How do cost aspects influence QMS?
Among the various expenses, personnel costs are significant expenditure, which includes training or hiring new staff such as quality assurance coordinators, project leaders, and task leads.
It is worth noting that the cost of staff time reduces significantly after the QMS has been implemented and matured. Another critical aspect to consider is time. A group in Barcelona reported that it took them 18 months to fully implement their QMS, despite already having a partial system in place.
References
- Centers for Disease Control and Prevention (CDC), Centers for Medicare & Medicaid Services (CMS), HHS. Medicare, Medicaid, and CLIA programs: laboratory requirements relating to quality systems and certain personnel qualifications: final rule. Fed Registry. 2003; 68:3640-3714.
- CMS State Operations Manual: Interpretive Guidelines Baltimore, MD: Centers for Medicare and Medicaid Services; 2004.
- Hooper, J. E., H. Richardson, A. W. Maters, K. C. Carroll, and P. J. Pronovost 2018, “The association of departmental quality infrastructure and positive change”: a pathology department illustration, Academic Pathology 5. Doi: 10.1177/2374289517744753.
- Vermaercke, P. 2000. Sense and nonsense of quality assurance in an R&D environment. Accreditation and Quality Assurance 5(1):11–15.
- Zapata-García, D., M. Llauradó, and G. Rauret. 2007. Experience in implementing ISO 17025 for the accreditation of a university testing laboratory. Accreditation and Quality Assurance 12(6):317–322.



