Capture Elisa:
ELISA works on the principle that specific antibodies bind the target antigen and detect the presence and quantity of antigens binding. To increase the sensitivity and precision of the assay, the plate must be coated with antibodies with high affinity.
- The capture ELISA is a common immunoassay technique used to detect and measure specific antigens or antibodies in biological samples.
- In capture ELISA, a solid phase (such as a microplate well) is coated with a capture antibody specific to the antigen of interest. After washing to remove any unbound antibody, a sample or standard containing the antigen is added, allowing the antigen to bind to the immobilized capture antibody.
- A detection antibody, labeled with an enzyme such as horseradish peroxidase or alkaline phosphatase, is then added to the well and incubated.
- Upon the addition of a substrate that reacts with the enzyme-labeled detection antibody, a signal proportional to the amount of antigen in the sample is produced.
- This signal is measured and the concentration of antigen in the sample is determined by comparing it to a standard curve of known antigen concentrations.
Capture antibodies are antibodies that are fixed or bound to a stable surface like a microplate, and are used to capture and bind specific antigens present in a biological sample.
Several examples of capture antibodies include:
Anti-HIV-1 p24 antibody: used for detecting HIV-1 p24 antigen in human serum or plasma, commonly used for HIV diagnosis.
Anti-CD3 antibody: used for identifying CD3 antigen on T cells, commonly employed in flow cytometry for analyzing T cell populations.
Anti-IL-6 antibody: used for detecting IL-6 cytokine in biological samples, typically used in ELISA assays for measuring IL-6 levels in plasma or serum.
Anti-PSA antibody: used for detecting prostate-specific antigen (PSA) in serum or plasma, frequently utilized for prostate cancer diagnosis.
Anti-CEA antibody: used for detecting carcinoembryonic antigen (CEA) in serum or plasma, widely used for colorectal cancer diagnostic assays.
Overall, capture antibodies play a crucial role in immunoassays, facilitating the detection of specific antigens in various biological samples. Numerous capture antibodies have been developed and are used in various diagnostic and research applications.
A principle in the selection of antibodies
Monoclonal antibodies are crucial in research, diagnostics, and therapy due to their high specificity. They are generated using hybridoma technology, which involves immunization, cell fusion, and antibody screening. Hybridoma cells producing the desired antibody are cloned through a time-consuming and laborious process. Different selection strategies have been developed to simplify this process and reduce time and effort. In this chapter, various selection strategies are presented and compared to conventional limited dilution technology.
Antibodies play a crucial role in pathogen detection, and antibody-based sensors are highly effective for rapid and sensitive analysis. The quality of the antibody used is critical, and recombinant antibodies offer advantages due to their genetic modification potential for improved selectivity, sensitivity, and immobilization.
The development of assays is simplified by the appropriate selection of antibodies and the design of the assay format.
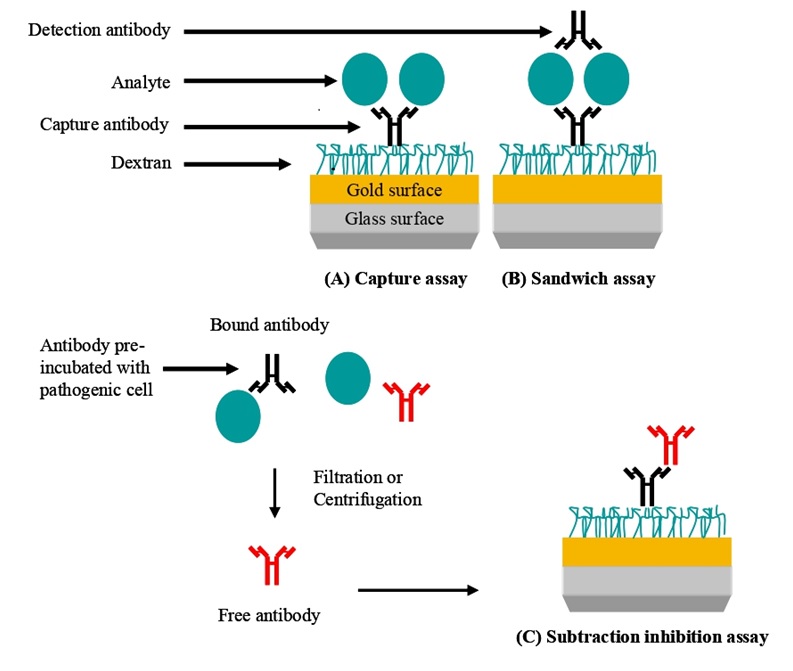
SPR-based assays for pathogen detection. (A) A specific antibody is immobilized and is used to capture the pathogen leading to a signal. (B) Pathogen or pathogen-related antigen is captured. Specificity is conferred by the binding of a second antibody. (C) The specific antibody reacts with the pathogen or pathogen-related antigen. Non-bound (free) antibody is isolated and detected when bound to an immobilized antibody (normally an anti-species antibody) on the chip. In this case, the signal generated is inversely proportional to the pathogen concentration.
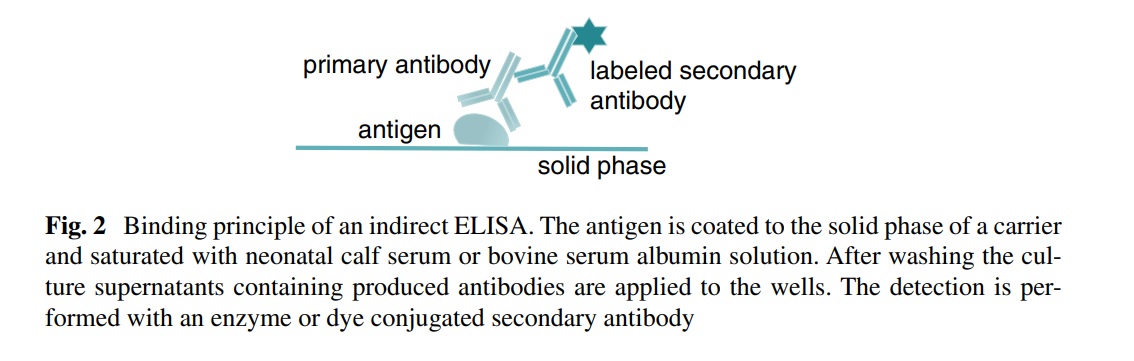
The test specimen is added; incubation allows antibodies in the test specimen to bind to the antigen. A secondary enzyme-labeled antibody is used to detect the test antibody. A chromogenic substrate is added; the enzyme converts the substrate to a detectable colored compound. The amount of color that develops is proportional to the amount of antibody in the test specimen.
ELISAs are not as effective as RT-PCR for early diagnosis of Lassa fever, but they can be useful for diagnosing patients in later stages. The IgG ELISA is particularly helpful for epidemiological studies and clinical trials, as it has a high specificity and is easier to operate, and has a higher throughput rate compared to IFA.
The study used an antibody capture ELISA to measure IgM antibody titers in paired sera from patients with encephalitis, dengue hemorrhagic fever (DHF), and Japanese encephalitis (JE). Diagnostic criteria were established based on titer distribution and ratios to distinguish between JE and dengue virus infections. These criteria successfully diagnosed the majority of patients with JE and dengue virus infections in the study.
Detection antibodies
- The primary detection antibody is an antibody that specifically recognizes and binds to the target protein or antigen of interest in a sample. This antibody is typically not conjugated with an enzyme or other detection molecule.
- On the other hand, the secondary detection antibody is an enzyme-conjugated antibody that recognizes and binds to the primary detection antibody, rather than the target antigen directly.
- This secondary antibody is used to amplify the signal and detect the presence of the primary antibody that is bound to the target antigen.
- Detection antibodies are specific antibodies used in immunoassays to bind and detect the captured antigen. There are several examples of detection antibodies, including anti-human IgG antibodies used to detect human IgG antibodies in diagnostic assays for diseases like COVID-19 and anti-PSA antibodies used to detect the prostate-specific antigen in diagnostic assays for prostate cancer.
- Anti-HRP antibodies can detect the presence of horseradish peroxidase in various immunoassays, while anti-HIV-1 gp41 antibody is used in diagnostic assays for HIV infection.
- Finally, the anti-IL-6 antibody is used to detect the captured IL-6 cytokine in various immunoassays. The selection of the appropriate detection antibody depends on the specific antigen being detected and the immunoassay format being used. Detection antibodies are crucial for accurate diagnosis in clinical and research applications.
Principles in selecting capture antibodies
- Select an antibody that is specific to the target antigen of interest
- Choose an antibody with high affinity to the target antigen
- Ensure that the antibody can bind the antigen in the sample matrix being used (e.g. blood, saliva, tissue)
- Consider the sensitivity and specificity of the assay, as well as the desired limit of detection and dynamic range
- Verify that the antibody can be effectively immobilized on the chosen solid support, such as a microplate or bead
- Check for potential interference from endogenous substances in the sample matrix that may interfere with the assay’s performance
- Consider the availability and cost of the antibody, as well as any ethical or regulatory considerations
- Validate the assay by testing against known positive and negative samples, and compare results to established reference methods if available.
These are some general principles to keep in mind when selecting capture antibodies for immunoassays.
Principles in selecting Detection antibodies
- The selection of a detection antibody is determined by several factors such as the properties of the target analyte, the sensitivity and specificity of the antibody, and the intended use of the assay.
- The detection antibody must have high specificity for the target analyte to reduce the possibility of reacting with other molecules present in the sample.
- It should also be sensitive enough to detect the analyte at the required concentration levels. If the assay involves a sandwich ELISA format, the detection antibody should be compatible with the capture antibody.
- The antibody should also be validated for the specific assay format and sample type to ensure its performance characteristics, such as specificity and sensitivity, are suitable for relevant matrices.
- The availability and affordability of the detection antibody are also important factors to consider.
- Additionally, the detection antibody must generate a signal compatible with the detection method used and have a high signal-to-noise ratio. In summary, choosing a detection antibody requires careful consideration of several factors to ensure the accuracy and reliability of the assay.
References
- Enzyme-linked immunosorbent assay (ELISA), Claire Horlock, Imperial College London, UK
- “Antibodies and Selection of Monoclonal Antibodies”, Katja Hanack, Katrin Messerschmidt & Martin Listek
- Antibody-Based Sensors: Principles, Problems, and Potential for Detection of Pathogens and Associated Toxins Barry Byrne , Edwina Stack, et.al
- “Immunoassays”, Sandeep K. Vashist, John H.T. Luong, in Handbook of Immunoassay Technologies, 2018
- “Development and evaluation of antibody-capture immunoassays for detection of Lassa virus nucleoprotein-specific immunoglobulin M and glycine”, Martin Gabriel, Donatus , Adomeh, Jacqueline Ehimuan et.al
- “Antibody-capture ELISA for detection of immunoglobulin M antibodies in sera from Japanese encephalitis and dengue hemorrhagic fever patients”, K. Bundo, A. Igarashi



