Introduction
The US Food and Drug Administration (FDA) requires that ELISA protocols be validated for drug development applications. The process of validation in the ELISA protocol is concerned with demonstrating the accuracy, reliability, and reproducibility of the assay for its intended purpose. This is achieved through testing the protocol with appropriate samples and evaluating its performance in terms of various parameters, such as limit of detection, linearity, accuracy, precision, specificity, and robustness.
The ultimate aim of validation is to ensure that the ELISA protocol meets the required standards set by regulatory agencies like the FDA and is suitable for its intended use. By conducting validation, drug developers can be assured of the quality and dependability of their results and uphold the safety and effectiveness of their drug products.
After careful protocol optimization, the determination of validation characteristics and the acquirement of an appropriate standard reliable, and inexpensive analytical method useful in diagnostics, research, or biomedicine, in general, can be achieved.
Designing the ELISA Protocol
The ELISA protocol should be designed to meet the requirements of the intended use and must be specific, sensitive, and reproducible. The protocol should include the type of ELISA (direct, indirect, sandwich), the antigen, antibody, and conjugate used, the assay format, the incubation time and temperature, and the method of data analysis.
Optimization
The ELISA protocol must be optimized to ensure that it is specific, sensitive, and reproducible. Optimization involves testing different conditions, such as antigen concentration, antibody concentration, and incubation time, to identify the optimal conditions that produce the most accurate and reliable results.
The biochemical activity of therapeutic antibodies can be examined by recovery ELISA and their residual activity can be determined. Thus, further individualization of therapy with biologics is possible using this test which seems to be suitable to diminish side effects and reduce costs.
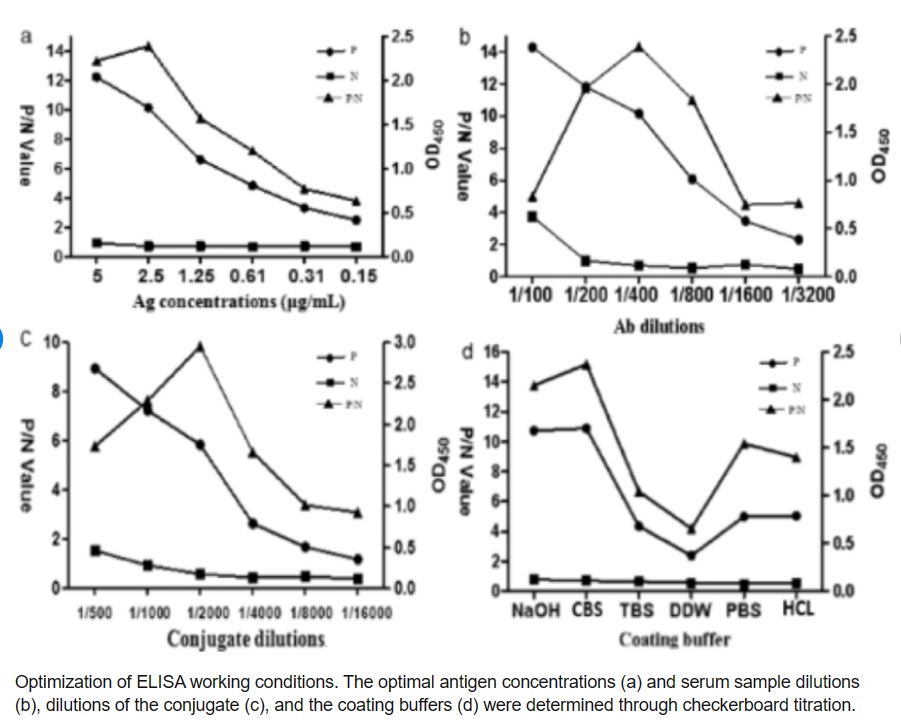
Validation
Validation involves testing the protocol using samples that are representative of the intended use. The validation should include determining the limit of detection, linearity, accuracy, precision, specificity, and robustness of the protocol. The validation must be performed according to the FDA’s guidelines for analytical method validation.
A study validated an ELISA protocol for measuring plasma oxytocin in phocid seals and compared different sample handling protocols. The vacutainer type used for collecting raw plasma affected the detected concentrations, likely due to anticoagulant interaction. However, extracted plasma concentrations were not affected. The capture and restraint protocol used affected raw plasma concentrations, likely due to stress response, highlighting the importance of standardized sample handling protocols. [6]
Documentation
All the steps involved in designing, optimizing, and validating the ELISA protocol must be documented. The documentation should include the protocol, the optimization results, the validation results, and any deviations from the protocol. The documentation must be kept in a secure location and made available to regulatory authorities upon request.
Monitoring and Maintenance
Once the ELISA protocol has been validated, it must be monitored and maintained to ensure that it continues to produce accurate and reliable results. This involves regular testing of control samples, monitoring of reagents and equipment, and regular calibration of equipment.
Inter-laboratory comparison
The US Food and Drug Administration (FDA) recommends conducting inter-laboratory comparison studies for ELISA assays to ensure the reproducibility and reliability of the assay across different laboratories. The inter-laboratory comparison involves comparing the results of the ELISA assay between different laboratories that follow the same protocol and use blinded samples.
Conclusion
The validation of an ELISA protocol for drug development as per FDA regulations is a crucial step in ensuring the accuracy, reliability, and reproducibility of the assay. By following these steps, drug developers can ensure that the ELISA protocol they use is validated and meets the requirements of the FDA.
References
- “Optimization, Validation, and Standardization of ELISA”, Rajna Minic and Irena Zivkovic”, September 2nd, 2020 Reviewed: October 5th, 2020 Published: December 3rd, 2020, DOI: 10.5772/intechopen.94338.
- FDA, editor. Guidance for Industry Bioanalytical Method Validation. U.S.D.o.H.a.H. Services. Rockville, Maryland: Food and Drug Administration (FDA); 2001
- Jaki T, Allacher P, Horling F (2016) A false sense of security? Can a tiered approach be trusted to accurately classify immunogenicity samples? J Pharm Biomed Anal 128:166–173 4.
- Jani D, Marsden R, Mikulskis A, Gleason C, Klem T, Krinos Fiorotti C, Myler H, Yang L, Fiscella M (2015) Recommendations for the development and validation of confirmatory anti-drug antibody assays. Bioanalysis 7(13):1619–1631
- Optimization of Omalizumab Dosage in Patients with Severe Persistent Allergic Asthma Using recoveryELISA Jens-Oliver Steiß & G. Becher ,BioDrugs volume 28, pages445–450 (2014).
- “Validation of an enzyme-linked immunoassay (ELISA) for plasma oxytocin in a novel mammal species reveals potential errors induced by sampling procedure”, Kelly J. Robinson a, Neil Hazon b, Mike Lonergan a, Patrick P. Pomeroy a, Journal of Neuroscience Methods Volume 226, 15 April 2014, Pages 73-79
- Kelly J. Robinson, Neil Hazon, Mike Lonergan, Patrick P. Pomeroy, Validation of an enzyme-linked immunoassay (ELISA) for plasma oxytocin in a novel mammal species reveals potential errors induced by sampling procedure, Journal of Neuroscience Methods,



