- In 1990, the US Congress funded the Ethical, Legal, and Social Implications (ELSI) Research Program as part of the Human Genome Project.
- The goal was to support research on the ethical, legal, and social implications of genetic and genomic research
- ELSI research has had a significant impact on subsequent scientific and public policy, the conduct of genomic research, and the implementation of genomic medicine
- ELSI research has influenced the way consent forms for genomic studies are drafted, policies and governance mechanisms for biobanks/repositories, understanding of risk perception, clarification of the meaning of race in genomic research, and intellectual property law surrounding genomics
- There is increasing recognition of the value of ELSI research, and there are calls for ELSI researchers to collaborate with genomics researchers and embed ELSI research within genomic research projects
- The term “ELSI research” has become familiar and is used to describe ethical, legal, and social research in other domains beyond genomics.
- However, the methods employed in some ELSI research, particularly normative and conceptual research, remain relatively unfamiliar to researchers in the basic biomedical and translational sciences.
Ethics of Genome Sequencing
Some points are discussed concerning the ethics of genome sequencing,
- ELSI research uses both empirical and nonempirical research methods.
- Conceptual research examines the meaning of various terms and ideas to increase conceptual clarity and argues that some understandings are better justified than others
- Normative research makes arguments based on value commitments supported by empirical evidence and assesses their consistency, utility, scope, and fit with other value commitments and empirical data
- ELSI research does not result in immutable accounts of what “is” or provide guidance for action that remains unchanged over time Instead, ELSI research yields guidance for action that evolves as conditions change, new information is discovered, and better arguments are made. Similarly, scientific research that seeks to discover facts and explain phenomena also evolves as new data and insights are gained.
- The ELSI Program has played a significant role in shaping policies related to the use of genomic information in clinical settings. Early studies funded by the program, focused on ethical and social issues related to population-based screening, carrier testing, prenatal testing, newborn screening, and predictive testing for both early and late-onset conditions. These studies contributed to the development of a variety of policy documents, recommendations, and guidelines issued by professional organizations and policy-making bodies.
Informed Consent
One of the major challenges they recognize is the need for international ethics harmonization in the requirements of informed consent and privacy protection. These requirements are underscored by international initiatives, including the International Cancer Genome Consortium (ICGC)
There is a growing ethical agreement that when researchers publish information on family history in the context of data release, they have a greater responsibility to address concerns and safeguard the privacy of relatives.
The American Society of Human Genetics (ASHG) recommends an ethical approach to the disclosure of genetic risk. They state that unauthorized disclosure of genetic risk can only be justified if attempts to encourage the patient to disclose have failed and if the harm is highly likely to occur and is serious, imminent, and foreseeable. Additionally, the at-risk relative(s) must be identifiable, and the disease in question must be preventable, and treatable, or early monitoring can reduce the genetic risk according to medically accepted standards.
The way information is provided and consent is obtained in DTC (Direct –To- Customer) genetic testing raises concerns about whether consumers fully understand what they are agreeing to. It is possible that consumers may not have read or fully comprehended the information presented to them. This raises questions about whether their decision to undergo testing is truly informed. Similar issues have been raised in the context of online transactions, where users may agree to terms of service without fully reading or understanding them.
The informed consent process requires understandable information to be provided to consumers about testing, along with ensuring the legal and cognitive capabilities of individuals to give their agreement. It also protects against involuntary testing.
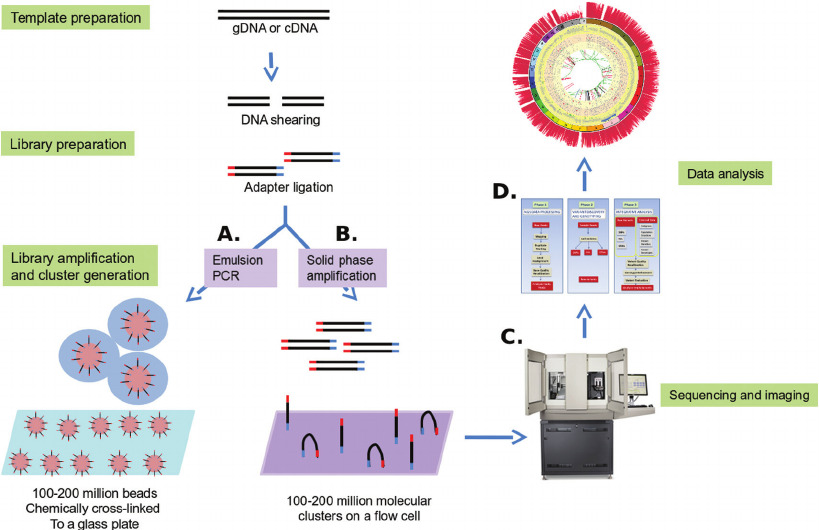
To account for the uncertainty of genomic information and the instability of digital networks, the consent form should include a disclaimer stating that privacy cannot be guaranteed. Additionally, it should provide information on data release and sharing, including the possibility of data being disseminated in public databases and the potential for re-identification of anonymized data.
Privacy Concerns
The concerns about various issues such as informed consent, data privacy, incidental findings management, and data availability to other researchers were developed.
Genetic privacy refers to the idea of protecting personal privacy when it comes to the collection, use, sharing, and disclosure of information related to an individual’s genetic makeup. This includes preventing unauthorized storage, manipulation, sharing, or public display of genetic data with third parties without the individual’s explicit consent.
In addition to safeguarding personal data related to genetic information, genetic privacy also encompasses protecting the ability to identify an individual based on their genetic sequence and preventing the use of genetic data to infer specific characteristics about a person, such as their susceptibility to certain diseases or their ancestral background. The idea is to ensure that individuals have control over who has access to their genetic data and how it is used.
The public release of genomic sequence information from participants in large-scale research studies has raised concerns about the privacy of these individuals. There have been instances where it has been demonstrated that previously anonymous participants in genetic studies can be identified using publicly available gene sequence data. This has led to questions about the adequacy of current privacy protections and the need for improved safeguards to protect the confidentiality of participants’ genetic data.
To address the growing concerns and issues related to genetic privacy, the United States has implemented regulations and policies at both the federal and state levels. These measures aim to provide legal protections to individuals and ensure that genetic information is used appropriately and with informed consent.
Data Breaches
Data breach refers to the unauthorized or accidental loss, alteration, disclosure, or access to personal data, which violates its security. Genomic data is not immune to security breaches and adversarial attacks. Vulnerabilities in the security of public databases containing genetic data have been revealed, which pose a threat to genetic privacy. Research subjects’ identities can sometimes be identified through their DNA alone. Removing patient identifiers is a common practice to protect anonymity in genomic medicine, but de-identified data is not afforded the same legal protections as research subjects. There is an increasing ability to re-identify patients and their genetic relatives from their genetic data, which raises concerns about genetic privacy.
The use of genetic information combined with social media and public data sources poses a risk to privacy, such as through voter registries and web searches. The ownership of genomic data is a legal concern, with instances of personal genome information being exploited or indirectly identifying family members.
These privacy concerns, including genetic discrimination, loss of anonymity, and psychological impacts, have been recognized by the academic community and government agencies.
Psychological Impacts: In a systematic review of perspectives on genetic privacy, researchers noted that individuals have concerns about their genetic information, including potential dangers and effects on themselves and their family members.
Regulations and Guidelines: Regulating access to genetic information can prevent insurance companies and employers from obtaining such data, which can help avoid discrimination. Breaches in genetic privacy could result in an affected individual losing their job or insurance.
Ethical Issues
The ethical concerns related to the use of genome-wide sequencing in children were identified and some of them are as follows,
- Ethical concerns related to genome-wide sequencing in children include challenges with the infrastructure, such as potential inconsistencies due to varying bioinformatics pipelines. Another challenge is interpreting the large amount of data generated and applying it in a clinical context. There is also the question of whether specific disease-associated genes should be actively searched for in every genome-wide sequencing.
- The ethical concerns related to pretest counseling for genome-wide sequencing in children involve the challenges of obtaining informed consent, such as determining what topics should be discussed during counseling, the potential impact of results over time, and the effect on parent-child relationships. Other issues include deciding whether there should be different types of consent based on urgency and the challenges of parental decision-making. Another concern is determining how much control parents should have over the types of findings received.
- The discussion of ethical concerns in genome-wide sequencing is particularly challenging, especially for non-geneticists, due to confusing terminology. This includes confusing language related to the test procedure, the scope of analysis, and the topic of uncertain findings.
Conclusion
One way to regulate the utilization of genetic information is by safeguarding its privacy. However, legislation aimed at preserving “genetic privacy” presents its challenges.
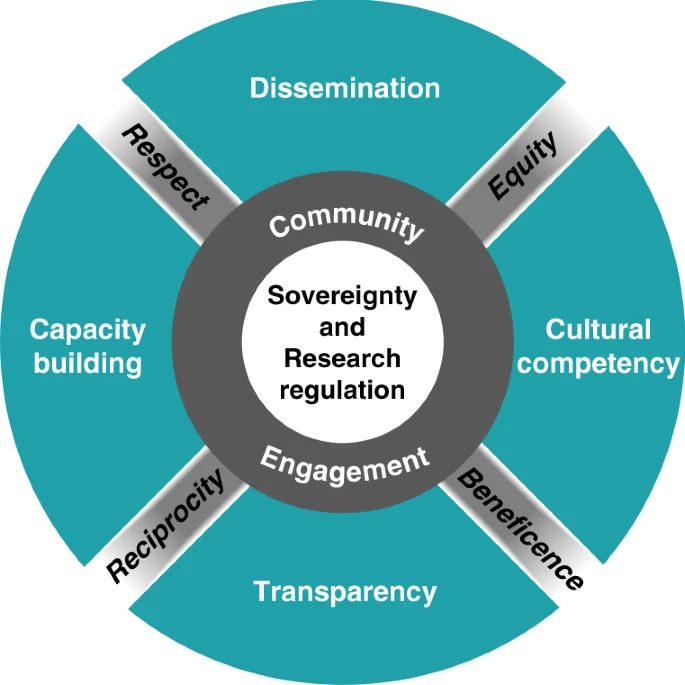
Ethical framework for enhancing genomic research with
Indigenous communities.
As members of Indigenous communities and scientists associated with the Summer Internship for Indigenous Peoples in Genomics (SING) Consortium, the authors have developed an ethical framework for engaging Indigenous communities in genomic research. This framework consists of six principles, informed by CBPR approaches, to promote ethical research practices, enhance the inclusion of diverse groups in genomic research, and build trust. The six principles are as follows: understand existing regulations, foster collaboration, build cultural competency, improve transparency, support capacity, and disseminate research findings. This ethical framework goes beyond the current US federal requirements for biomedical and behavioral research and emphasizes the importance of tribal research regulations and benefits to participants and their communities.
References
- McEwen JE, Boyer JT, Sun KY, et al. The Ethical, Legal, and Social Implications Program of the National Human Genome Research Institute: reflections on an ongoing experiment. Annu Rev Genomics Hum Genet. 2014;15:481–505.
- Ancillotti M, RerimassieV, Seitz SB, Steurer tryptophan, “An update of public perceptions of synthetic biology: Still undecided? NanoEthics “‘, 2016;10: 309-325.
- Murphy BJ, Bridges JF, Mohamed A, Kaufman aspartic acid, “Public preferences for the return of research results in genetic research: a conjoint analysis”, Genet Med. 2014;16: 932–9.
- Anderson RT, Press N, Tucker DC, Snively BM, Wenzel L, et al. 2005, “Patient acceptability of genotypic testing for hemochromatosis in primary care”, Genet. Med. 7:557–63.
- Callanan NP, Cheuvront BJ, Sorenson JR. 1999,” CF carrier testing in a high-risk population: anxiety, risk perceptions, and reproductive plans of the carrier by “non-carrier” couples”, Genet. Med. 1:323–27.
- Botkin JR,” Protecting the privacy of family members in survey and pedigree research”, JAMA. 2001;285:207–211.
- National Institutes of Health. Protection of Third Party Information in Research: Recommendations of the National Institutes of Health to the Office of Human Research Protections. [online],< http://bioethics.od.nih.gov/nih_third_party_rec.html>(2001).
- Maronick T.J., “Do consumers read terms of service agreements when installing software? A two-study empirical analysis”, Int. J. Bus. Soc. Res. 2014;4:137–145.
- “European Society of Human Genetics Statement of the ESHG on direct-to-consumer genetic testing for health-related purposes”, Eur. J. Hum. Genet. 2010;18:1271–1273. doi: 10.1038/ejhg.2010.129.
- El Mustapha Bahassi, e, al;, iNext-generation sequencing technologies: Breaking the sound barrier of human genetics, September 2014Mutagenesis 29(5):303-310DOI:10.1093/mutage/geu031.
- Chow-White P.A., MacAulay M., Charters A., Chow proline, “From the bench to the bedside in the big data age: ethics and practices of consent and privacy for clinical genomics and personalized medicine”, Ethics Inf. Technol. 2015 doi: 10.1007/s10676-015-9373-x.
- Adam S., Friedman J.M., “Individual DNA samples and health information “, sold by 23andMe. Genet. Med. 2015;10–11 doi: 10.1038/gim.2015.82.
- “From genetic privacy to open consent” (PDF). Nature Reviews Genetics. Retrieved 8 November 2019.
- Shi, Xinghua; Wu, Xintao (January 2017). “An overview of human genetic privacy: An overview of human genetic privacy”. Annals of the New York Academy of Sciences. 1387 (1): 61–72. doi:10.1111/nyas.13211. PMC 5697154. PMID 27626905.
- Genetic privacy”. Nature. 493 (7433): 451. 24 January 2013. doi:10.1038/493451a. PMID 23350074.
- “Florida becomes the first state to enact DNA privacy law, blocking insurers from genetic data”. Washington Examiner. 2020-07-02. Retrieved 2020-07-06.
- De Cristofaro, Emiliano (2012-10-17). “Whole Genome Sequencing: Innovation Dream or Privacy Nightmare?”. arXiv:1210.4820 [cs.CR].
- Clayton, Ellen W.; Halverson, Colin M.; Sathe, Nila A.; Malin, Bradley A. (2018-10-31). “A systematic literature review of individuals’ perspectives on privacy and genetic information in the United States”. PLOS ONE. 13 (10): e0204417. Bibcode:2018PLoSO..1304417C. doi:10.1371/journal.pone.0204417. ISSN 1932-6203. PMC 6209148. PMID 30379944.
- Anderlik, Mary R; Rothstein, Mark A. (2001). “Privacy and Confidentiality of Genetic Information: What Rules for the New Science?”. Annual Review of Genomics and Human Genetics. 2: 401–433. doi:10.1146/annurev.genom.2.1.401. PMID 11701656.
- Wouters RHP, Cornelis C, Newson AJ, Bunnik EM, Bredenoord AL, “Scanning the body, sequencing the genome: dealing with unsolicited findings”, Bioethics. 2017;31(9):648–56. https://doi.org/10.1111/bioe.12375.
- . Annas, G.J., Glantz, L.H., Roche, P.A., 1995. The Genetic Privacy Act and Commentary. Boston University School of Health, Boston, MA.
- Malhi, R. S. & Bader, A. Engaging Native Americans in genomics research. Am. Anthropol. 117, 743–744 (2015).



