Antibody-based precision medicine is a type of therapy that utilizes monoclonal antibodies to specifically target disease-causing molecules. Monoclonal antibodies are laboratory-made antibodies designed to bind to a specific target with high affinity and specificity. The use of monoclonal antibodies as therapeutic agents has been an important advancement in precision medicine. By targeting specific molecules involved in the development or progression of a disease, monoclonal antibodies can provide highly targeted and effective therapies with fewer side effects compared to traditional treatments.
The development of monoclonal antibodies involves selecting a target molecule involved in the disease process, generating an antibody that specifically binds to that target, and then testing the antibody for efficacy and safety in preclinical and clinical studies. Once approved, monoclonal antibodies can be administered to patients either alone or in combination with other therapies, such as chemotherapy or radiation.
Precision medicine is especially crucial in autoimmune disorders, which are heterogeneous and demonstrate both clinical and molecular variability. Determining the most effective treatment approach for individual patients may be more intricate and significant in autoimmune diseases than in other illnesses. Precision medicine relies on two critical components: patient stratification and the application of targeted treatments. Nevertheless, there is a paucity of investigation and implementation of precision medicine in systemic autoimmune disorders.
Recently, there has been a demonstration of the potential of precision medicine through the use of biological disease-modifying antirheumatic drugs based on peripheral immune cell phenotyping in the clinical management of psoriatic arthritis. However, the practical application of precision medicine in real-world clinical settings has yet to be fully explored. Despite this, there are promising signs that precision medicine is on the horizon. For example, a preclinical study using a small animal model conducted by the Perelman School of Medicine at the University of Pennsylvania and published in Nature Biotechnology showed that the groundbreaking chimeric antigen receptor (CAR) T cell therapy, which is typically used to treat specific types of cancer, can be repurposed to treat a variant of the autoimmune disease known as myasthenia gravis (MG). This new product, called MuSK chimeric autoantibody receptor T cells or MuSK-CAART, has been developed to accurately target the cause of muscle-specific tyrosine kinase (MuSK)-MG.
Overview of Autoimmune Diseases and Targeted Immunotherapy
Autoimmune diseases are pathological states that involve an abnormal inflammatory response against self-antigens and affect a significant portion of the general population, ranging from 3% to 10%. In the United States, autoimmune diseases impact more than 80 million people, accounting for roughly 10% of all chronic illnesses. There are more than 80 identified autoimmune diseases, such as rheumatoid arthritis, multiple sclerosis, lupus, and type 1 diabetes. These conditions can affect any part of the body and can present in various degrees of severity. The exact causes of autoimmune diseases are not fully understood, but it is believed that a combination of genetic and environmental factors plays a role. Certain genetic variations can increase the risk of developing an autoimmune disease, and environmental factors such as infections, toxins, and stress can trigger the immune system to attack healthy cells.
Conventional treatment for autoimmune diseases typically involves medications that suppress the immune system, such as corticosteroids, immunosuppressants, and biologic drugs. These medications can help to control the symptoms of the disease and prevent further damage to the affected tissues.
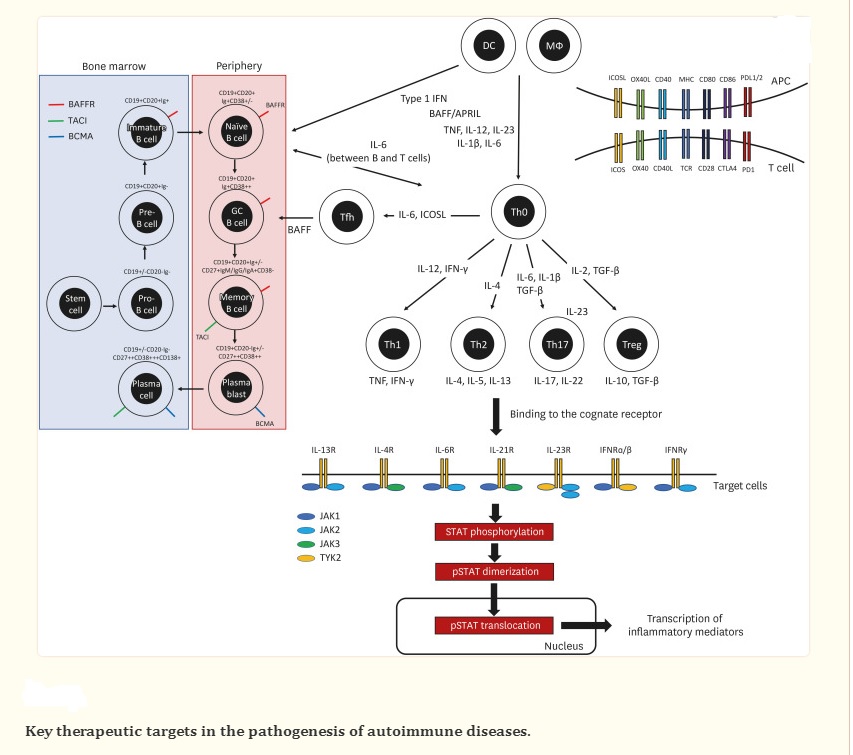
Advancements in comprehending disease pathogenesis and novel techniques for drug production have resulted in the widespread usage of targeted immunotherapy to treat autoimmune diseases. The emergence of recombinant protein therapeutics like monoclonal antibodies and receptor-antibody fusion proteins has been made possible by advanced molecular engineering, targeting soluble mediators or cell surface markers. Since the approval of selective protein therapeutics for targeting TNF in rheumatoid arthritis (RA) in the 1990s, targeted immunotherapies have revolutionized the treatment of autoimmune diseases. The top-selling drug globally for several years, as reported by the Global Pharmaceuticals Market Report, has been adalimumab, followed by other targeted immunotherapies like pembrolizumab, ibrutinib, and ustekinumab.
Advances in Antibody Therapies and Targeted Immunotherapy for Autoimmune Diseases
As our understanding of autoimmune disease continues to improve and biotechnology advances, a growing number of drugs with innovative therapeutic targets and improved efficacy are being developed, offering promising results. One of the earliest antibody therapies approved by the FDA for immunosuppression therapy against transplant rejection was the anti-CD3 antibody Muromonab-CD3, which is a murine IgG2 antibody (clone OKT3). While it has the potential for treating autoimmune diseases like MS, clinical trials were halted due to toxicity, specifically an allergic reaction to mouse antibodies.
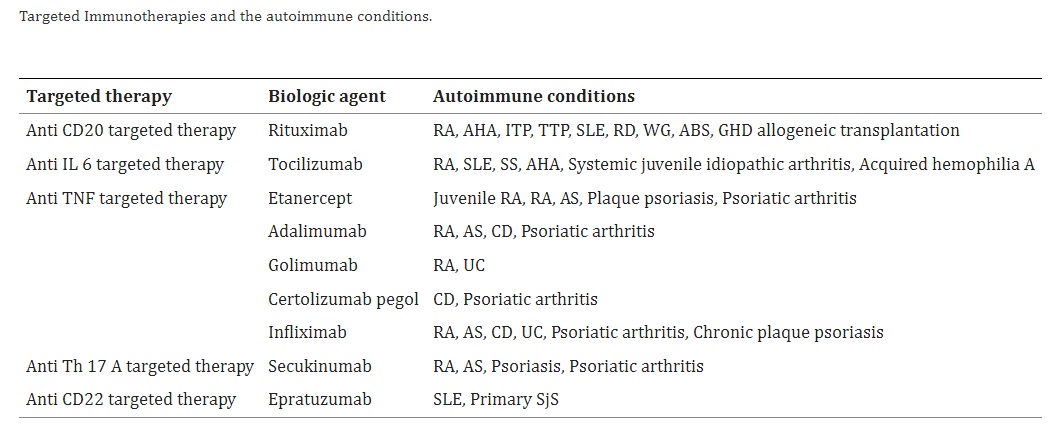
There are several antibody treatments available for various autoimmune diseases, some of which are listed below.
- For MS treatment, two drugs have been approved: Natalizumab, an anti-VLA-4 antibody, and Alemtuzumab, an anti-CD52 antibody. However, there have been reports of a potential association between natalizumab treatment and melanoma risk. Alemtuzumab can remove CD52-positive leukocytes, mainly T and B cells, but it may also lead to the development of unique secondary autoimmune diseases, although the risk of secondary infection and malignancy is low.
- Anti-CD20 antibody therapies that target B cells producing autoantibodies or activating T cells have also been used to treat some autoimmune diseases. Rituximab, one of these antibodies, was approved for treating rheumatoid arthritis (RA) in 2006, while Ocrelizumab was approved for treating MS.
- Targeted Immunotherapies such as anti-TNF antibodies, including infliximab, adalimumab, and etanercept, have revolutionized the treatment of autoimmune diseases. Infliximab, initially approved in 1998 for Crohn’s disease, received FDA approval in 1999 for the treatment of RA in combination with methotrexate. Currently, infliximab is approved for the treatment of RA, ankylosing spondylitis (AS), psoriatic arthritis (PsA), Crohn’s disease (CD), and ulcerative colitis (UC).
- Tocilizumab (TCZ) is a type of monoclonal antibody that has been genetically engineered to target and bind to the interleukin-6 receptor (IL-6R) in humans. Its primary purpose is to treat rheumatoid arthritis, as well as systemic juvenile idiopathic arthritis (sJIA) and polyarticular juvenile idiopathic arthritis (pJIA).
- Ixekizumab: Randomized controlled trials targeting IL-17 can be an effective way to treat Ankylosing spondylitis (AS), as it plays a significant role in the disease’s pathogenesis. Two recent studies have found that ixekizumab, a monoclonal antibody that targets IL-17a, is both highly effective and safe in treating AS.
With the growing understanding of how diseases develop, there is a surge in the development of biological drugs that target the pathways responsible for inflammation associated with inflammatory diseases. After the successful implementation of biologic therapies for treating autoimmune diseases, molecular targets have expanded to include intracellular kinases. There is great interest in blocking convergent signals using small molecule kinase inhibitors for their potential in providing effective therapy and long-term safety.
Challenges of Antibody-Based Precision Medicine in Autoimmune Diseases
The use of precision medicine to treat autoimmune diseases poses certain difficulties compared to its application in cancer treatment. Firstly, the development of targeted drugs for autoimmune diseases has been slower historically and the number of available treatments is limited. Secondly, it is more challenging to obtain tissue biopsies from patients with autoimmune diseases as compared to those with cancer.
Although antibody-based precision medicine has shown promising results in the treatment of autoimmune diseases, several challenges still need to be addressed to fully realize its potential. Some of the major challenges of antibody-based precision medicine in autoimmune diseases include:
Patient heterogeneity: Autoimmune diseases are complex disorders that can present differently in different patients. This can make it difficult to identify the specific targets for antibody-based precision medicine, as not all patients will respond to the same therapy.
Development of resistance: As with any therapy, there is a risk of patients developing resistance to antibody-based precision medicine over time. This can limit the long-term effectiveness of these therapies and may require the development of new therapies or combination therapies.
Cost: Antibody-based precision medicine can be expensive, both for patients and for healthcare systems. This can limit access to these therapies for some patients, particularly those in lower-income countries.
Side effects: While targeted therapies can have fewer side effects than traditional immunosuppressive drugs, they are not without risk. Some patients may experience side effects such as infusion reactions, allergic reactions, or infections.
Limited availability of approved therapies: While several antibody-based precision medicines are approved for the treatment of autoimmune diseases, there are still many disorders for which there are no approved therapies. This highlights the need for continued research and development in this area.
Lack of biomarkers: There is currently a lack of biomarkers that can predict which patients will respond to antibody-based precision medicine. This can make it difficult to identify the patients who are most likely to benefit from these therapies.
Need for personalized dosing: Antibody-based precision medicine often requires personalized dosing based on a patient’s characteristics and disease status. This can be challenging to implement in clinical practice, particularly in resource-limited settings.
The Perspective of Further Development of Precision Medicine
A study that analyzed RNA-sequencing data from blood samples identified two distinct 23-gene transcriptional signatures that can be used to differentiate responders to TNF-i or rituximab. In the case of rheumatoid arthritis, differences in a chromosome conformation signature in blood have been identified as predictive markers of methotrexate treatment at baseline. Liquid biopsies are also expected to provide important insights into disease pathogenesis and contribute to precision medicine. If the consistent clinical benefits of treatment strategies based on biopsied-tissue (synovium) information, liquid biopsy, and other methods are validated, they could be valuable in real-world clinical settings for practicing precision medicine in systemic autoimmune diseases.
In the future, advancements in genomic sequencing and analysis technologies will enable healthcare professionals to gain a deeper understanding of genetic variations that contribute to disease. As a result, we can expect to see the emergence of new targeted therapies that are designed to treat specific genetic mutations or disease subtypes.
Another area of focus for the further development of precision medicine is the integration of large-scale data analysis and artificial intelligence (AI). By analyzing vast amounts of patient data, including genomic information, clinical history, and lifestyle factors, AI algorithms can identify patterns and associations that would be difficult for humans to detect. This has the potential to accelerate the development of new treatments and diagnostic tools, as well as improve patient outcomes. Although precision medicine has already made significant strides in the diagnosis and treatment of various diseases, there is still much to be explored and developed to fully realize its potential. Here are some potential areas of further development in precision medicine:
Personalized Treatment Approaches: Precision medicine aims to provide tailored treatments based on a patient’s genetic makeup, environment, and lifestyle. To further develop personalized treatment approaches, more research is needed to identify genetic and non-genetic factors that affect disease development and progression.
Gene Editing Technologies: Gene editing technologies like CRISPR/Cas9 have the potential to revolutionize precision medicine by enabling precise and targeted modifications of genes. This can lead to the development of new therapies for genetic diseases and improved outcomes for patients with cancer and other diseases.
Multi-Omics Data Integration: The integration of genomic, transcriptomic, proteomic, and metabolomic data can provide a more comprehensive view of disease mechanisms and drug responses. Developing new computational and statistical methods for analyzing and interpreting large-scale data sets will be critical to unlocking the full potential of multi-omics data integration.
Novel Therapeutic Targets: The identification of novel therapeutic targets is essential for the development of effective treatments for diseases that are currently untreatable or have limited treatment options. Advances in genomic technologies and computational biology are providing new insights into disease mechanisms, which can help identify new therapeutic targets.
Advanced Diagnostics: Developing more accurate and comprehensive diagnostic tools is crucial for precision medicine. This includes the use of advanced imaging technologies, liquid biopsies, and other non-invasive diagnostic tests.
Patient Involvement: Patient involvement in precision medicine research and clinical trials is crucial for ensuring that treatments are effective and meet patients’ needs. Incorporating patient perspectives and experiences into the development of precision medicine can lead to better patient outcomes.
Ethical Considerations: As precision medicine advances, ethical considerations must be addressed, including issues related to data privacy, informed consent, and equity in access to care.
References
- Miyagawa I and Tanaka Y (2022) Dawn of Precision Medicine in Psoriatic Arthritis. Front. Med. 9:851892. doi: 10.3389/fmed.2022.851892
- https://www.pennmedicine.org/news/news-releases/2023/january/car-t-like-treatment-for-rare-form-of-autoimmune-disease
- Jung SM, Kim WU. Targeted Immunotherapy for Autoimmune Disease. Immune Netw. 2022 Feb 17;22(1):e9. doi: 10.4110/in.2022.22.e9. PMID: 35291650; PMCID: PMC8901705.
- Masahiro Yasunaga, Antibody therapeutics and immunoregulation in cancer and autoimmune disease, Seminars in Cancer Biology, Volume 64, 2020, Pages 1-12, ISSN 1044-579X, https://doi.org/10.1016/j.semcancer.2019.06.001.
- Nahra V, Hasbani GE, Chaaya M, Uthman I. The Use of Infliximab (Remicade®) for the Treatment of Rheumatic Diseases at a Tertiary Center in Lebanon: A 17-Year Retrospective Chart Review. Mediterr J Rheumatol. 2020 Dec 22;31(4):400-405. doi: 10.31138/mjr.31.4.400. PMID: 33521572; PMCID: PMC7841097.



