Precision medicine, also referred to as “personalized medicine,” is a novel strategy for customizing disease prevention and management by considering variations in individuals’ genes, surroundings, and habits. The goal is to enhance health outcomes and minimize healthcare expenses by offering precise diagnoses, choosing efficient treatments, and decreasing unfavorable reactions to drugs.
Precision medicine has made technological strides with the emergence of “biopharmaceuticals,” which are quickly gaining ground over conventional pharmaceuticals in the market with innovations like gene therapy, immunotherapy, and antibody-drug conjugates. In comparison to traditional medications, biopharmaceuticals deliver various advantages such as reduced adverse reactions, greater precision, and efficacy. These drugs provide tailored treatments that hasten the recovery from illnesses that were once incurable using synthetic drugs. Monoclonal antibodies are the most profitable and are used to treat autoimmune diseases, angiogenesis-related diseases, cardiovascular diseases, inflammatory diseases, and cancer. The top-selling biopharmaceuticals in 2017 were mainly monoclonal antibodies, with eight out of the ten bestsellers falling into this category. The most financially successful drug among them was mAb adalimumab (ADA). This drug, a tumor necrosis factor-alpha (TNF-α) inhibitor has generated global sales of around USD 62.6 billion between 2014 and 2017. The number of newly registered monoclonal antibodies is also expected to grow and dominate the biopharmaceutical market.
Overview of Rheumatoid Arthritis
Rheumatoid arthritis (RA) is a chronic autoimmune disease affecting the joints and causing inflammation, pain, and stiffness. In RA, the immune system mistakenly attacks the synovial membrane, which lines the joints and produces synovial fluid that helps lubricate and cushion the joints. This leads to inflammation and damage causing pain, swelling, and stiffness. Over time, the joint damage can become irreversible, leading to deformity and disability. RA can also affect other organs and tissues in the body, such as the lungs, heart, and blood vessels. The range of symptoms can include fatigue, fever, and weight loss.
Rheumatoid arthritis is a frequently occurring autoimmune disease with an incidence rate of 20 cases per 100,000 people per year and a prevalence of over 500 cases per 100,000 people. In the United Kingdom, around 387,000 individuals were affected with rheumatoid arthritis, accounting for 0.8% of the adult population and over 2 million people in the United States.
Although the exact cause of RA is not fully understood, significant progress has been made in understanding it over the past 30 years. The triggers that cause immune tolerance to break and induce autoimmunity are currently unknown; however, having human leukocyte antigen (HLA) genes HLA-DR1 and HLA-DR4 are associated with a 50% risk of developing RA. Presently, 50 to 80% of RA patients have rheumatoid factor autoantibodies, which are typically IgM and IgA molecules, and/or anti-citrullinated peptide antibodies (ACPA). Studies have shown that the presence of ACPA is a reliable predictor of rapid disease progression, as defined by the destruction of joints.
There is currently no cure for RA, but early diagnosis and treatment can help manage symptoms and slow the progression of the disease. In addition, physical therapy and lifestyle changes, such as exercise and a healthy diet, can also be beneficial.
Monoclonal Antibodies in the Treatment of Rheumatoid Arthritis
MAbs have revolutionized the treatment of RA by targeting specific molecules involved in the inflammatory process. The development of mAbs for the treatment of RA began in the 1990s, and since then, several mAbs have been approved for use in patients with RA.
Before the introduction of monoclonal antibodies and fusion proteins for RA, a wide variety of small molecules were used to manage pain and inflammation in this disease. These included broad-spectrum anti-inflammatory drugs such as non-steroidal anti-inflammatory drugs (NSAIDs) and analgesics, as well as anti-malarials, anti-metabolites, alkylating agents, and especially glucocorticoids, which were used in various combinations. However, these drugs were frequently linked to significant side effects, which contributed to the burden of co-existing conditions that often affect RA patients.
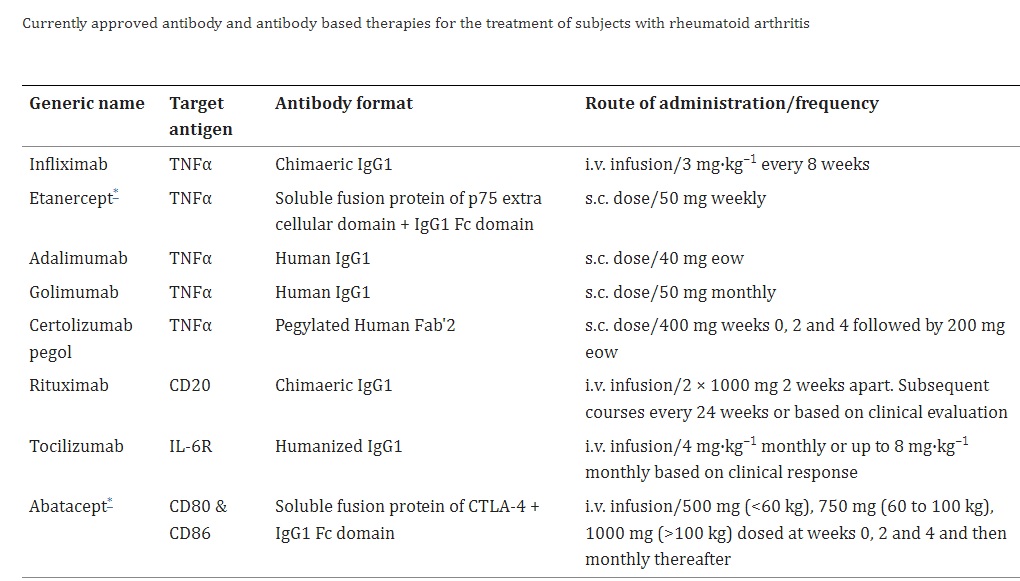
Feldmann and Maini conducted a study to examine the effectiveness of infliximab (cA2), a chimeric anti-tumor necrosis factor (TNF) antibody, in a small group of patients with RA. As a consequence, infliximab as the first monoclonal antibody for RA treatment was approved, with an acceptable safety profile. This breakthrough in using targeted biologic therapies to TNF for treating RA has become one of the biggest success stories in biologics, with a market now valued at over $8 billion per year worldwide and five anti-TNF molecules (infliximab, etanercept, adalimumab, golimumab, and certolizumab) approved either globally or in the USA. Additionally, the success achieved in utilizing anti-TNF therapies as a primary line of biologic treatment has enabled rheumatologists to evaluate and obtain approval for other antibody or fusion protein-based therapies, including rituximab (B cell depletion therapy), abatacept (inhibition of co-stimulation), and tocilizumab (anti-IL-6 receptor therapy).
The use of mAbs in the treatment of RA has significantly improved outcomes for patients with the disease by reducing symptoms such as joint pain and swelling, improving physical function, and slowing the progression of joint damage. However, mAbs can have side effects, including an increased risk of infections and malignancies, and may not be effective for all patients. As such, the use of mAbs in RA treatment is carefully monitored and individualized for each patient.
Next-Generation Antibodies for Rheumatoid Arthritis (RA)
Next-generation antibodies are a new class of biologic agents that are being developed for the treatment of RA. These antibodies are designed to be more specific and targeted than traditional biologic agents and to have fewer side effects.
The progress made in the engineering of antibodies has resulted in the creation of next-generation therapeutic antibodies, which consist of antibody fragments, dual/bispecific targeting variants, and immunocytokines. These antibodies are designed to be smaller, more stable, possess greater affinity and better tissue penetration, and display less immunogenicity and toxicity compared to traditional antibodies.
One example of a next-generation antibody is sarilumab, which targets the interleukin-6 receptor (IL-6R). IL-6 is a cytokine that is involved in the inflammatory process in RA, and blocking its receptor has been shown to reduce inflammation and improve symptoms in patients with RA. Sarilumab was approved by the FDA in 2017 for the treatment of moderate to severe RA. Another next-generation antibody is olokizumab, which is effective in reducing inflammation and improving symptoms in patients with RA in clinical trials and is currently being reviewed by regulatory agencies for approval.
Other next-generation antibodies that are being developed for the treatment of RA include those that target other cytokines and cell types involved in the inflammatory process, such as interleukin-17 (IL-17), interleukin-23 (IL-23), and CD40.
Ablynx, a Belgian biopharmaceutical company, has created novel dual-specific therapeutic nanobodies, ozoralizumab (ATN-103) and vobarilizumab (ALX0061), for treating RA. These are the first humanized dual-specific nanobodies that can neutralize TNF and IL-6R, respectively. In addition, they also attach to human serum albumin to increase their half-life. AbbVie has developed another bispecific antibody, Remtolumab (ABT-122), which targets two crucial human cytokines, TNF and IL-17A.
Challenges and Future Directions in Precision Medicine for Rheumatology
Several challenges need to be addressed to fully realize the potential of precision medicine in rheumatology. Some of these challenges and future directions for precision medicine in rheumatology are:
Biomarker identification and validation: One of the key challenges in precision medicine for rheumatology is the identification and validation of biomarkers that can be used to guide treatment decisions. Biomarkers are molecular or genetic signatures that can indicate the presence or severity of a disease, as well as predict response to treatment. Several biomarkers have been identified and validated for RA, including anti-citrullinated protein antibodies (ACPAs), rheumatoid factor (RF), and C-reactive protein (CRP). These biomarkers can be used to diagnose RA, predict disease progression, and monitor response to treatment. However, there is still a need for the identification and validation of new biomarkers, particularly for other rheumatologic diseases such as systemic lupus erythematosus (SLE) and psoriatic arthritis (PsA). This will require large-scale studies that combine genomic, transcriptomic, and proteomic data with clinical and imaging data to identify new biomarkers that are both sensitive and specific.
Data integration and analysis: Another challenge in precision medicine for rheumatology is the integration and analysis of large amounts of data from different sources. This includes genomic, transcriptomic, proteomic, and clinical data, as well as data from imaging and wearable devices. To make sense of this data and identify patterns and correlations that can inform treatment decisions, there is a need for advanced computational tools and algorithms that can integrate and analyze these different types of data. This will require collaboration between rheumatologists, bioinformaticians, and data scientists to develop and validate these tools.
Patient stratification: Precision medicine requires the stratification of patients into different subgroups based on their disease phenotype, genetic profile, and other factors that may influence response to treatment. This allows for the development of more targeted and personalized treatments that are tailored to the specific needs of individual patients. However, patient stratification is still a challenge in rheumatology, particularly for diseases like SLE and PsA that have heterogeneous clinical manifestations and variable responses to treatment. This will require the development of new clinical and imaging tools that can better define disease subtypes and predict response to treatment.
Cost-effectiveness: Precision medicine can also help improve outcomes and reduce healthcare costs by identifying patients most likely to benefit from a certain treatment and avoiding treatments that are unlikely to be effective. However, the high cost of biologic agents, genetic testing and other diagnostic tools can be a barrier. To address this challenge, there is a need for studies comparing the cost of precision medicine with the cost of traditional treatments and evaluating the long-term impact of precision medicine on healthcare costs and patient outcomes.
Ethical and legal considerations: Finally, the ethical and legal considerations need to be addressed in the development and implementation of precision medicine in rheumatology. This includes issues related to data privacy and ownership, informed consent, and equitable access to care.
References
- Campbell J, Lowe D, Sleeman MA. Developing the next generation of monoclonal antibodies for the treatment of rheumatoid arthritis. Br J Pharmacol. 2011 Apr;162(7):1470-84. doi: 10.1111/j.1476-5381.2010.01183.x. PMID: 21182494; PMCID: PMC3057286.
- Lim SH, Kim K, Choi CI. Pharmacogenomics of Monoclonal Antibodies for the Treatment of Rheumatoid Arthritis. J Pers Med. 2022 Jul 31;12(8):1265. doi: 10.3390/jpm12081265. PMID: 36013214; PMCID: PMC9410311.
- Tanaka T, Hishitani Y, Ogata A. Monoclonal antibodies in rheumatoid arthritis: comparative effectiveness of tocilizumab with tumor necrosis factor inhibitors. Biologics. 2014 Apr 7;8:141-53. doi: 10.2147/BTT.S37509. PMID: 24741293; PMCID: PMC3984066.
- Senolt L. Emerging therapies in rheumatoid arthritis: focus on monoclonal antibodies. F1000Res. 2019 Aug 30;8:F1000 Faculty Rev-1549. doi: 10.12688/f1000research.18688.1. PMID: 31508202; PMCID: PMC6719675.



