Antibodies are proteins produced by B cells that can identify and neutralize foreign organisms or antigens. They are made up of two heavy and two light chains, and their structure determines their function. Monoclonal antibodies are made in the lab and are typical of the IgG isotype, which has a long half-life in the body. Murine mAbs were the first to be developed, but they had significant drawbacks such as allergic reactions, short half-life, and poor recruitment of effectors function. Human mAbs have been developed to overcome these limitations and are being used as therapeutics, particularly in oncology indications. To overcome the limitations of murine mAbs, chimerical and humanized mAbs were developed, with humanized mAbs showing the most promise. Fully human mAbs were generated using phage display technology and transgenic mice, which have significantly reduced immunogenic potential and behave similarly to human endogenous IgGs. Advances in understanding factors that influence mAb immunogenicity have led to the development of tools to reduce clinical immunogenicity through deselection or deimmunization.
Advantages Over Traditional Therapies
Monoclonal antibodies have several advantages over traditional therapies. They are highly specific and can be designed to target a particular antigen, reducing off-target effects. Additionally, they have a long half-life and can be administered as infrequently as once a month. Monoclonal antibodies can also be produced at a large scale and with high purity, which enables their widespread use. Finally, monoclonal antibodies can be engineered to enhance their potency and pharmacokinetic properties, making them more effective than traditional therapies.
Mechanisms of Action: How do monoclonal antibodies work?
Therapeutic anti-CD3 mAb
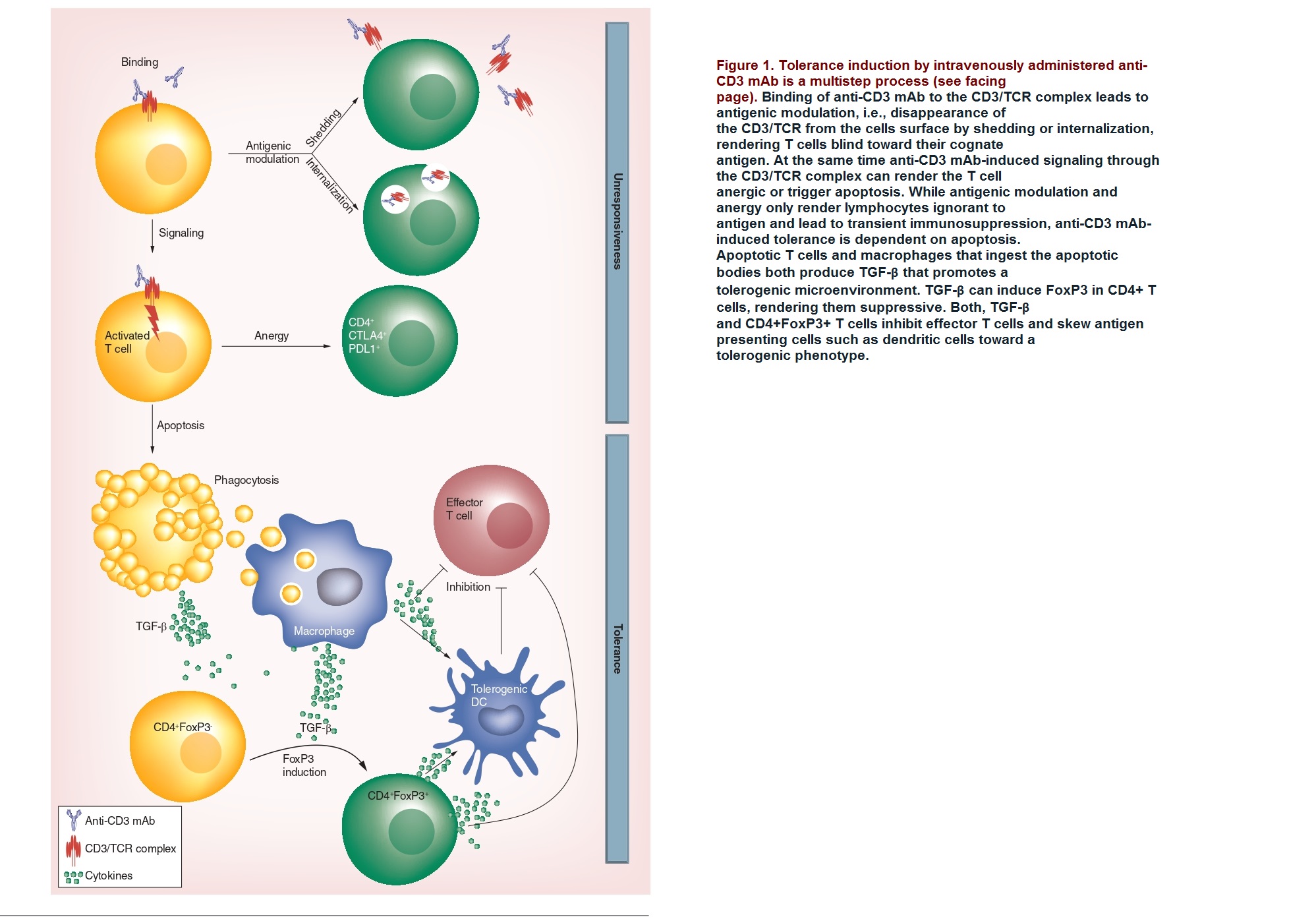
The therapeutic potential of anti-CD3 monoclonal antibodies (mAb) in treating autoimmune diseases such as Type 1 diabetes and inflammatory bowel disease (IBD) were studied. Anti-CD3 mAb therapy is thought to induce tolerance in T cells by various mechanisms, including the modulation of the CD3/T-cell receptor complex, induction of anergy or apoptosis in activated T cells, and an increase in Tregs, which are responsible for maintaining immune tolerance.
Immune Response against SARS-CoV-2
The immune response against SARS-CoV-2 involves both innate and adaptive immune systems. The production of antibodies, a process initiated by effectors B cells during the immune phase, plays a key role in neutralizing the virus. The regulation of antibody production is a complex process controlled by cytokines, low-molecular-weight proteins, peptides, and glycoproteins secreted by immune cells to regulate immune cell function. During an innate immune response, pattern recognition receptors on immune cells recognize and bind Pathogen-Associated Molecular Patterns (PAMPs), which can stimulate the production of several proinflammatory cytokines. These include Interleukin (IL)-1, Tumor Necrosis Factor (TNF)-α, Interferon Gamma (IFN-γ), IL-6, and C-X-C motif chemokine ligand 10 (IFN-γ-induced protein 10).
The induction of CD4+ cells is an important factor in the production of granulocyte-macrophage colony-stimulating factor (GM-CSF) and proinflammatory cytokines. NK cells and cytotoxic T (CD8+) lymphocytes are crucial in the fight against viral infection, as their functional collapse has been shown to correlate with disease progression. The role of antibodies in protecting against SARS-CoV-2 infection is still not fully understood. While virus-specific antibodies are generally associated with antiviral immunity, the link between antibody levels and symptom severity in asymptomatic individuals is yet to be established. Moreover, IgG antibodies against SARS-CoV-2 are observed to degrade rapidly during early infection. Developing neutralizing antibodies against the virus could be useful in treating COVID-19, but monoclonal antibodies are prone to degradation and loss of stability during manufacturing. However, using neutralizing monoclonal antibodies is still an important therapeutic approach, as shown in fig.2.

Development and Approval: How are monoclonal antibodies tested and approved?
To address the limitations of reduced effectiveness and immunogenicity associated with traditional antibodies, researchers have developed methods to modify rodent antibodies to more closely resemble human antibodies while maintaining their ability to bind to target molecules. The first example of this, a chimerical antibody called abciximab, was approved by the FDA in 1994 for use in inhibiting platelet aggregation in cardiovascular disease.
Preclinical studies
Pharmaceutical and biotechnology companies are highly interested in the development of mAbs, but safety assessments often require non-human primates (NHPs), which raises ethical, scientific, and economic concerns. To address this issue, an expert working group composed of representatives from leading companies, research organizations, and institutes in Europe and the USA has analyzed data on mAbs for various therapeutic areas to identify opportunities to reduce the use of NHPs. They recommend scientifically appropriate development pathways and study designs for potential mAbs. The addendum of ICHS6 presents an opportunity for the scientific and regulatory community to adopt strategies that minimize the use of NHPs and increase the efficiency of mAb development.
Clinical trials phases I-III
Clinical trials of therapeutic monoclonal antibodies typically go through three phases:
Phase I: This phase is conducted on a small group of healthy volunteers to assess the safety and pharmacokinetics of the antibody, such as how it is absorbed, distributed, metabolized, and excreted by the body.
Phase II: In this phase, the antibody is tested on a larger group of patients to determine its efficacy and optimal dosage for treating a particular condition. Safety continues to be monitored in this phase as well.
Phase III: This phase is typically the final stage of clinical trials and involves a larger patient population to further evaluate the safety and efficacy of the antibody in a controlled, randomized manner. The results of this phase are submitted to regulatory agencies for approval before the antibody can be marketed for clinical use.
Regulatory agencies and requirements
The regulatory process for approving new drugs involves balancing the benefits of making them available with the potential safety risks. Clinical trials and preclinical studies, along with product design and manufacture, are crucial for successful drug development. The FDA (Food and Drug Administration) and ICH (International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use) publish guidance documents for drug development, including mAbs. The clinical drug development process is divided into four phases: Phase 1, to identify a safe and active dose; Phase 2, to obtain preliminary estimates of treatment-mediated effects; Phase 3, to establish clinical benefit; and Phase 4, conducted after market approval, to address additional questions about safety and efficacy. The types of Phase 4 studies are agreed upon between the FDA and the sponsor and form post-marketing commitments.
Post-marketing surveillance
Post-marketing surveillance or pharmacovigilance is necessary to monitor the safety and efficacy of monoclonal antibodies once they are approved for use in the market. The process aims to detect any rare or long-term adverse effects that were not observed during clinical trials and collect data on the drug’s effectiveness in real-world settings. The FDA requires manufacturers to conduct post-marketing surveillance to monitor adverse events associated with the drug, while the FDA also conducts its surveillance through the Adverse Event Reporting System (AERS). The process involves collecting and analyzing data from spontaneous reports of adverse events, post-approval clinical trials, and epidemiological studies. Post-marketing surveillance is an ongoing process, and any new safety concerns that arise may lead to changes in the product label or even withdrawal of the drug from the market.
The Challenges and Advancements in the Manufacturing and Formulation of Therapeutic Antibodies
Therapeutic antibodies are a crucial technology in the biopharmaceutical industry, as they can be tailored to create specialized treatments, including targeted drug delivery systems and increased bioavailability. However, challenges still exist in the manufacturing and formulation of these antibodies. They are susceptible to degradation and have poor tissue penetration, requiring high concentrations for effective doses. Stability is also a major concern, as it affects manufacturing yield and formulation considerations. Despite these challenges, advancements in discovery, manufacture, and formulation technologies have led to improvements in antibody therapies. Computational tools and nanocarrier technologies have shown promise in improving stability and potentially allowing for non-invasive administration routes. Overall, the refinement of antibody design and formulation strategies has the potential to overcome these challenges and develop superior treatment options.
Next-generation technologies
Recent advances in genome research have been made possible by the development of next-generation sequencing (NGS) techniques and improved bioinformatic data analysis. NGS allows for the reading of millions of gene variants simultaneously, which is especially important for highly variable genes such as Ig genes. The CDR-H3 domain of the antibody heavy chain alone can have a repertoire of up to 106 to 107 clonotypes, with the repertoire of VH: VL clonotypes being even more diverse. The use of NGS in mAb production has already been reported in several cases.
Personalized Medicine Strategies for Improving Monoclonal Antibody Therapy in Inflammatory Bowel Diseases (IBD)
Monoclonal antibody therapies have greatly improved treatment for chronic inflammatory diseases like Crohn’s disease and ulcerative colitis. However, there is variability in patient response due to differences in pharmacokinetics and drug exposure. Personalized medicine, involving monitoring drug exposure and adjusting dosing for individual patients, is gaining acceptance as a way to optimize mAb therapy for better outcomes in IBD. Implementation of personalized medicine through clinical decision-support tools can aid in guiding therapeutic decision-making for IBD patients.
References
- Chatenoud L, “CD3-specific antibodies restore self-tolerance: mechanisms and clinical applications”, Curr. Opin. Immunol. 17(6), 632–637 (2005).
- Kuhn C, Weiner HL. Therapeutic anti-CD3 monoclonal antibodies: from bench to bedside. Immunotherapy. 2016 Jul; 8(8):889-906. Doi: 10.2217/imt-2016-0049. Epub 2016 May 10. PMID: 27161438.
- Penaranda C, Tang Q, Bluestone JA. Anti-CD3 therapy promotes tolerance by selectively depleting pathogenic cells while preserving regulatory T cells. J. Immunol. 187(4), 2015–2022 (2011).
- Ablamunits V, Bisikirska B, Herold KC., “Acquisition of regulatory function by human CD8 (+) T cells treated with anti-CD3 antibody requires TNF”, Eur. J. Immunol. 40(10), 2891–2901 (2010).
- Sifniotis V, Cruz E, Eroglu B, Kayser valine,”Current Advancements in Addressing Key Challenges of Therapeutic Antibody Design, Manufacture, and Formulation. Antibodies “, (Basel). 2019 Jun 3; 8(2):36. Doi: 10.3390/antib8020036. PMID: 31544842; PMCID: PMC6640721.
- Lavinder J.J., Horton A.P., Georgiou G., et al. 2015, “Next-generation sequencing and protein mass spectrometry for the comprehensive analysis of human cellular and serum antibody repertoires”, Curr. Opin. Chem.Biol. 24, 112–120.
- Lushova AA, Biazrova MG, Prilipov AG, Sadykova GK, Kopylov TA, Filatov AV. Next-Generation Techniques for Discovering Human Monoclonal Antibodies. Mol Biol. 2017;51(6):782-787. Doi: 10.1134/S0026893317060103. Epub 2017 Dec 14. PMID: 32214477; PMCID: PMC7088925.