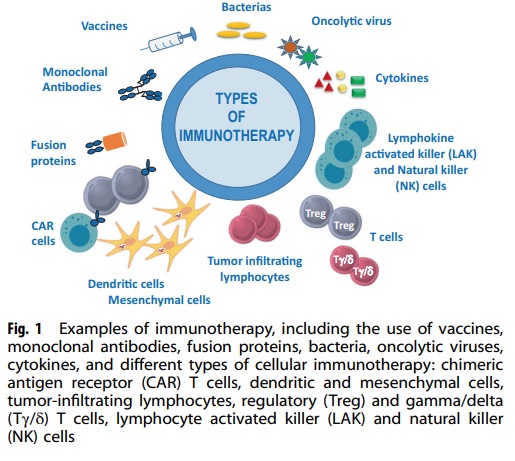
Immunotherapy is a medical treatment that harnesses the power of the immune system to combat diseases like cancer and autoimmune disorders. This form of therapy stimulates the immune system to identify and destroy cancer cells or other irregular cells. By doing so, it aids the immune system in recognizing and fighting off these diseases. In the late 20th century, the recognition of immunity to bacterial infections led to the idea that tumors also possess distinct antigens capable of eliciting host reactions. However, all animal experiments performed at that time involved transplantable tumors in randomly-bred or recently captured wild animals, which invalidated these experiments because of the element of allograft rejection. Despite this problem, many groups of clinicians attempted to use various immunological methods as “magic bullets” for the treatment of advanced cancer patients. The earliest attempts were poorly documented and mostly anecdotal, but the first adequate series was described in 1895. The potential methods of tumor immunotherapy can be divided into 6 main groups for ease of discussion.
Monoclonal antibodies (mAbs) are antibodies that can bind to the same epitope, and they are produced by homogeneous hybrid cells that are clones of the same parent cell. In contrast, polyclonal antibodies are made up of several different immune cells. Hybridomas are created by fusing an antibody-producing B cell with a myeloma cell. The resulting hybrid cells produce antibodies of a single specificity, making them mAbs. The production of mAbs was first reported in 1975 by Köhler et al using hybridoma technology.
Klein’s immunotherapy for skin and mucosal tumors involves promoting a delayed hypersensitivity reaction with agents like dinitrochlorobenzene and then challenging the tumor area with the same agent. Tumors are highly sensitive to this reaction, resulting in inflammation and regression. This method is successful for basal cell carcinoma, but it’s unclear if it targets tumor antigens. Other agents like BCG and vaccinia injected into tumors also regress, likely due to intratumoral inflammation rather than immunological targeting. Immunotherapy involves using components of the immune system to treat cancer and autoimmune diseases, as well as using vaccines to prevent and treat infectious and allergic diseases.
A Brief Overview of Monoclonal Antibodies and their Role in Immunotherapy
A molecular biology diagnosis is not sufficient to evaluate the progress of COVID-19 or identify old infections and humoral immunity. This has created a demand for rapid and simpler immunity-based tests. Immunological tests are advantageous over molecular biology techniques as they are quick, precise, and allow for differentiation between old and recently infected cases.
Immunological tests measure the immune response to SARS-CoV-2 and use components of the immune response as parts of the test. Enzyme-based immunoassays use specifically manufactured monoclonal antibodies to detect the antigen, which can be found in body fluids like oropharyngeal swabs, saliva, sputum, blood, urine, or stool.
Recently, immunological tests have been used to identify SARS-CoV-2 proteins using monoclonal antibodies against multiple forms of N protein or S protein (S1, S2). Monoclonal antibodies against SARS-CoV-2 nucleocapsid proteins could serve as a foundation for a future rapid antigen detection test. Immunological tests are crucial in COVID-19 epidemiology, vaccine development, and immune response.
Intrathecal Administration of Monoclonal Antibodies for Leptomeningeal Carcinomatosis
Leptomeningeal carcinomatosis is a difficult condition to treat, with limited options available such as supportive care, radiation, or intrathecal chemotherapy. The efficacy of conventional therapies is poor, and the blood-brain and blood-cerebrospinal barriers hinder the accumulation of drugs at the site of the disease. Monoclonal antibodies such as trastuzumab and rituximab are first-line agents for their respective diseases, but their efficacy is limited in leptomeningeal carcinomatosis due to their inability to penetrate the barriers. Intrathecal administration of these agents has shown promise and may be safe and effective. Trastuzumab was tolerated at doses ranging from 5 to 100 mg, and rituximab was tolerated at doses ranging from 10 to 30 mg. More data is needed to determine the optimal doses, frequency, and duration of therapy for intrathecal administration of these agents. This approach is investigational and should only be considered in a patient’s refractory to other available treatments.
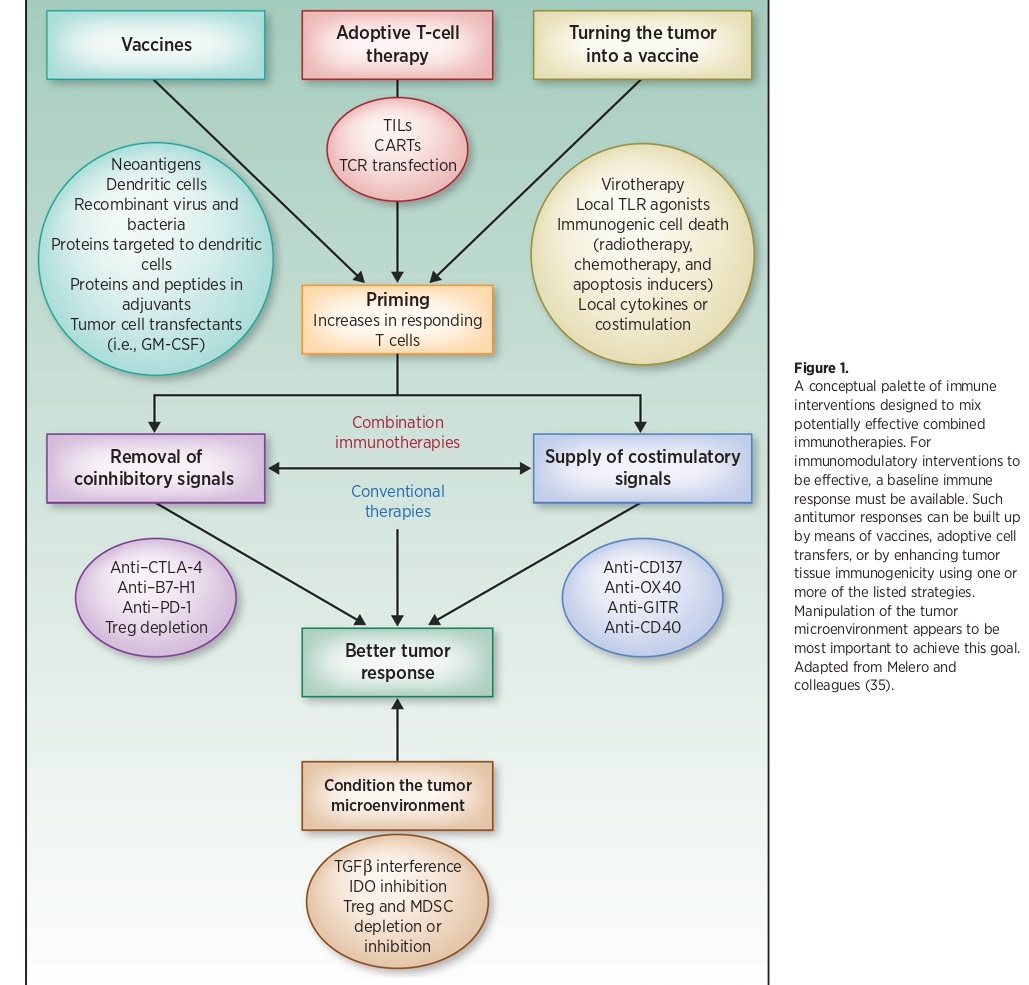
To enhance the effectiveness of immune interventions, a basic immune response needs to be present. This response can be built up using various strategies such as vaccines, adoptive cell transfers, or by increasing the immune response of tumor tissues. These strategies can be combined to create potentially effective combined immunotherapies. The manipulation of the tumor microenvironment is crucial to achieving this goal.
The Use of Monoclonal Antibodies in the Treatment of Autoimmune Diseases
Autoimmune diseases are caused by the abnormal activation of T lymphocytes. Monoclonal antibodies (MAbs) can be used to block this activation by targeting various proteins involved in T cell function. For example, anti-CD4 MAbs inhibit T helper cell function, while anti-CD40-L MAbs block co-stimulation by preventing the binding of CD40 and CD40 ligands. Anti-TNF MAbs can also be used to block the release of tumor necrosis factor, which plays a role in the pathogenesis of autoimmune diseases.
The use of MAbs in the treatment of autoimmune diseases has become increasingly common in both adults and children and is often used as an alternative when other treatments have failed. MAbs are usually given intravenously in a hospital setting and are generally well-tolerated. Various MAbs have been used to treat different autoimmune diseases, such as acute anterior uveitis, idiomatic posterior uveitis, uveitis associated with Behçet’s disease, and chronic childhood arthritis refractory to other treatments. In Crohn’s disease, anti-TNF-alfa MAbs have been used with good results, and in chronic childhood arthritis, they have been used in combination with methotrexate. Side effects of MAbs are generally mild, with headaches and increased infections being the most commonly reported.
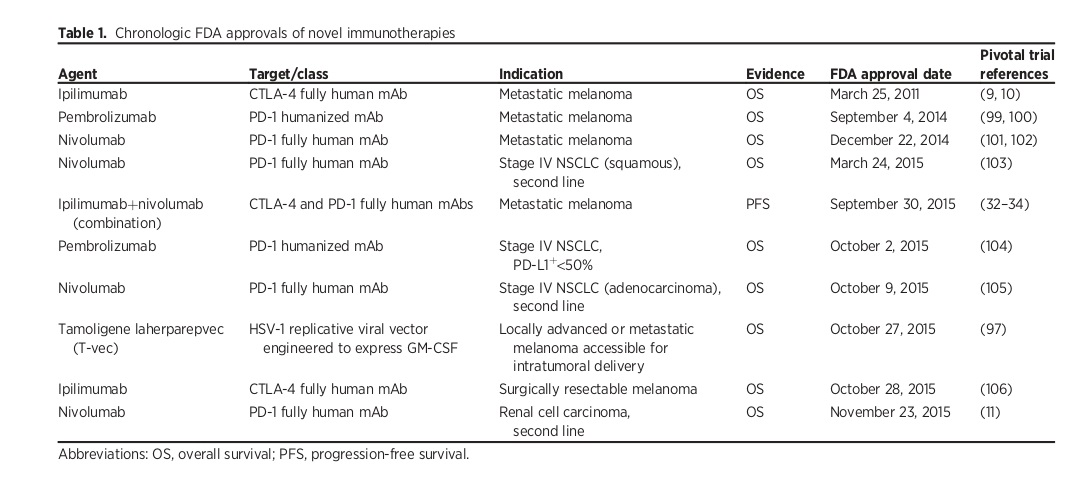
Immunotherapeutic synergy refers to the therapeutic effect that is greater than the sum of the effects of individual components in a combination. It is considered the most powerful tool for advancing immunotherapy. The first combination of immunotherapy treatments to receive FDA approval for metastatic melanoma involves the blocking of both CTLA-4 and PD-1.
Categorizing Immunotherapeutic Strategies for COVID-19
Immunotherapeutic strategies for COVID-19 fall into four categories. The first involves targeting inflammatory molecules, like cytokines, using monoclonal antibodies, receptor antagonists, or inhibitors to reduce the cytokine storm. The second strategy is passive immunotherapy using plasma from recovered patients. The third strategy is immunomodulation therapy with intravenous immunoglobulin to prevent secondary infections and inflammation. This approach has been successful in treating Kawasaki syndrome in children. The fourth strategy is cellular immunotherapy using SARS-Cov-2-specific T and NK cells and autologous or allogenic Tregs.
Monoclonal Antibodies as Immunotherapy in Infectious Diseases like COVID-19
The COVID-19 pandemic caused by the novel coronavirus SARS-CoV-2 has led to significant morbidity and mortality worldwide, and despite efforts to develop effective treatments, no therapeutic agent has been approved yet. Immunotherapy, such as convalescent plasma and monoclonal antibodies, has been effective against viral diseases in the past. Monoclonal antibodies are a type of passive immunotherapy that can be isolated from the blood of infected patients or engineered in the laboratory, and they offer a more specific form of therapy compared to convalescent plasma. While a safe and effective COVID-19 vaccine remains the best option to combat the pandemic, monoclonal antibodies can be a helpful intervention, especially in high-risk settings.
The production of proinflammatory cytokines and chemokines, including GM-CSF and IL-6, plays a central role in the immunopathogenesis of SARS-CoV-2. Multiple cytokines and chemokines are raised in severe cases of COVID-19, and anti-cytokine monoclonal antibodies (mAbs) could be effective in reducing mortality. Tocilizumab, an IL-6 inhibitor, has shown immediate improvement in severe and critical patients, and several international clinical trials are ongoing. Lenzilumab, a GM-CSF antagonist, has also shown promising results in reducing the risk of invasive ventilation and/or death in severe COVID-19 patients. Other anti-GMCSF mAbs, CCR5 antagonists, and mAbs against IL-1β, CD73, F. XIIa, connective tissue growth factor, VEGF, P-selectin, C5, IFNɣ, and IL-1 are also under investigation in clinical trials.
The Impact and Potential of Monoclonal Antibodies in Immunotherapy
Monoclonal antibodies have transformed the field of immunotherapy by providing a targeted approach to treating various types of cancer. By binding to specific antigens on cancer cells, they stimulate the immune system to attack them. They can also be customized to improve their effectiveness and combined with other treatments to create a multi-pronged approach to cancer treatment.
Looking ahead, ongoing research is focused on developing bispecific antibodies and personalized monoclonal antibody therapies that can be tailored to individual patients. There is also interest in expanding the use of monoclonal antibodies to other diseases such as autoimmune disorders and infectious diseases. With continued research, the potential for more effective and personalized treatments will continue to grow, giving hope for better outcomes for patients.
References
- Currie, G. A. (1972), “Eighty years of immunotherapy: a review of immunological methods used for the treatment of human cancer”, British Journal of Cancer, 26(3), 141.
- Perissinotti AJ, Reeves DJ. Role of Intrathecal Rituximab and Trastuzumab in the Management of Leptomeningeal Carcinomatosis. Annals of Pharmacotherapy. 2010; 44(10):1633-1640. doi:10.1345/aph.1P197.
- A. Martin-Mateos, “Monoclonal antibodies in Pediatrics: use in prevention and treatment”, Allergologia et Immunopathologia, Volume 35, Issue 4, 2007, Pages 145-150, ISSN 0301-0546, https://doi.org/10.1157/13108225.
- Zhand, M. Jazi, S. Mohammadi, R. Rasekhi, G. Rostamian, M. Kalani, et al., COVID-19: the immune responses and clinical therapy candidates Int, J. Mol. Sci. 21 (15) (2020), 5559. https://doi.org/10.3390/ijms21155559.
- Patterson, H. Seethamraju, K. Dhody, M. Corley, K. Kazempour, J. Lalezari, et al., “CCR5 inhibition in critical COVID-19 patients decreases inflammatory cytokines, increases CD8 T-cells, and decreases SARS-CoV2 RNA in plasma by day 14”, Int. J. Infect. Dis. 103 (2020) 25–32, https://doi.org/10.1016/j.ijid.2020.10.101.
- Whiteside TL, Demaria S, Rodriguez-Ruiz ME, Zarour HM, Melero I. “Emerging Opportunities and Challenges in Cancer Immunotherapy”, Clin Cancer Res. 2016 Apr 15; 22(8):1845-55. Doi: 10.1158/1078-0432.CCR-16-0049. PMID: 27084738; PMCID: PMC4943317.



