Monoclonal antibodies (mAbs) are proteins that can specifically bind to antigens. They have become increasingly popular in therapy, with numerous molecules approved by regulatory agencies. MAbs are made up of identical light and heavy chains that connect through disulphide bonds, with variable domains that bind to antigens and constant regions that interact with receptors on cell surfaces. Understanding the pharmacokinetics and immunogenicity of mAbs is essential for their functionality. Currently, over 500 antibodies are in the early stages of research, while more than 50 mAbs are in the final stages of clinical development, most of which are directed at fighting cancer and autoimmune or inflammatory diseases such as melanoma, lupus, and rheumatoid arthritis. Biosimilars of infliximab and rituximab are among those that have shown great clinical success.
The Evolution of Monoclonal Antibody-Based Therapies
Antibodies have been studied and used for over 200 years, with polyclonal antisera being used to treat some infectious diseases for many decades. Monoclonal antibodies (mAbs) were discovered in 1975 and quickly became valuable tools in pathology and laboratory investigation due to their ability to bind to specific antigenic epitopes. However, early clinical trials of mAb-based therapies for cancer were disappointing due to issues with administering murine mAbs to humans, including an immune response against the therapeutic mAb, rapid clearance, and suboptimal interactions with the human immune system. Nevertheless, persistent investigators continued to explore ways in which mAbs could be used in cancer treatment and developed techniques for genetic modification of murine mAbs to produce chimeric mouse-human mAbs. These modified mAbs behave immunologically like naturally occurring human IgG and have longer half-lives, better interaction with the human immune system, and can be administered on a practical schedule for patients. As a result, mAbs are now successfully used in cancer therapy by targeting cancer directly, altering the host response to cancer, delivering cytotoxic substances to cancer, and retargeting the cellular immune response toward cancer.
How Monoclonal Antibodies Work
The Immune System and COVID-19
The immune system is responsible for protecting the body from harmful substances, germs, and cell changes. White blood cells play a key role in the immune system and travel through the body to monitor for invading microbes. The lymphatic system is an important component of the immune system, with lymph nodes serving as specialized compartments where immune cells can encounter antigens. COVID-19 is an RNA virus that enters the body through respiratory droplets and binds to the ACE2 receptor using its spike protein. The virus replicates and can cause mild to severe disease, with age and co-morbidities being significant risk factors for severe disease and death. Reports suggest that patients aged over 60 years who have co-morbidities, especially hypertension, are at a higher risk for severe disease and death from COVID-19 infection.
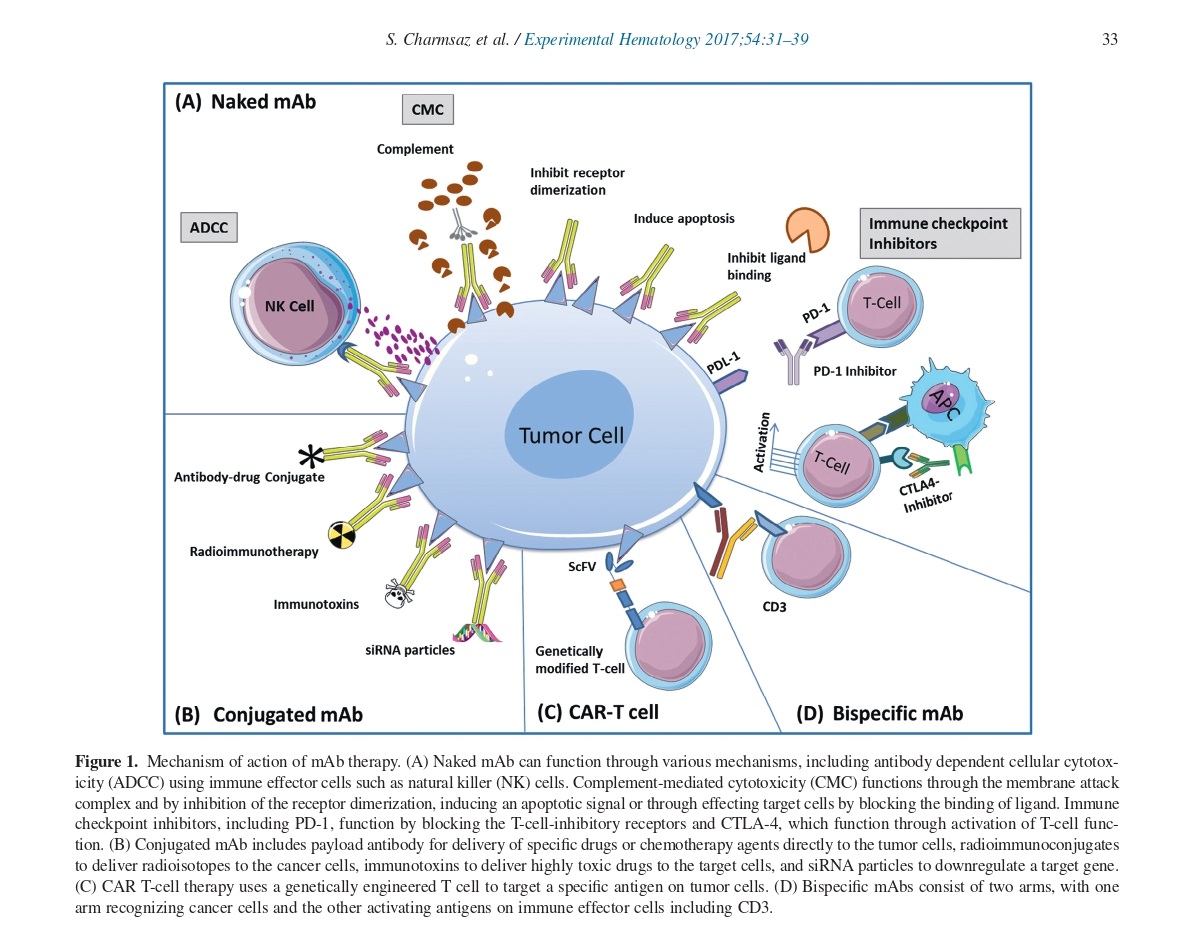
Mechanisms Action of mAb Therapies
MAb therapies have various mechanisms of action. Naked mAbs can directly affect cell function by inhibiting or activating signaling pathways or triggering apoptosis. They can also target immune effector cells like natural killer (NK) cells through antibody-dependent cellular cytotoxicity (ADCC) or complement-mediated cytotoxicity (CMC) by inhibiting receptor dimerization. Immune checkpoint inhibitors, such as PD-1 and CTLA-4, can activate T-cell function by blocking T-cell-inhibitory receptors.
Conjugated mAbs contain payload antibodies that deliver specific drugs or chemotherapy agents directly to tumor cells, immunotoxins that deliver highly toxic drugs, radioimmunoconjugates that deliver radioisotopes, and siRNA particles that down-regulate target genes. CAR T-cell therapy uses genetically engineered T-cells that target specific antigens on tumor cells. Bispecific mAbs consist of two arms, with one arm recognizing cancer cells and the other activating antigens on immune effectors cells, including CD3.
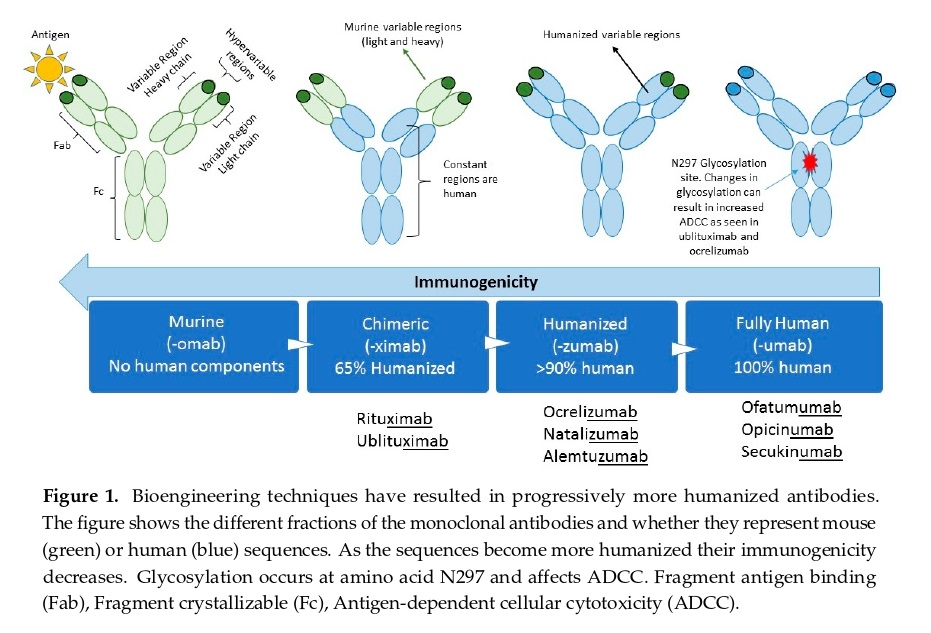
Development of Therapeutic mAbs: From Murine to Chimeric Mouse-Human Antibodies
Therapeutic mAbs were initially derived from non-human species like mice, and the first approved mAb was muromonab-CD3 (Orthoclone OKT3), which targeted the CD3 surface protein of T lymphocytes to prevent organ rejection in 1985. However, repeated exposure to murine-based proteins was associated with the development of antidrug antibodies (ADAs), causing reactions. To minimize the immunogenicity of murine mAbs, chimeric mouse-human antibodies were developed.
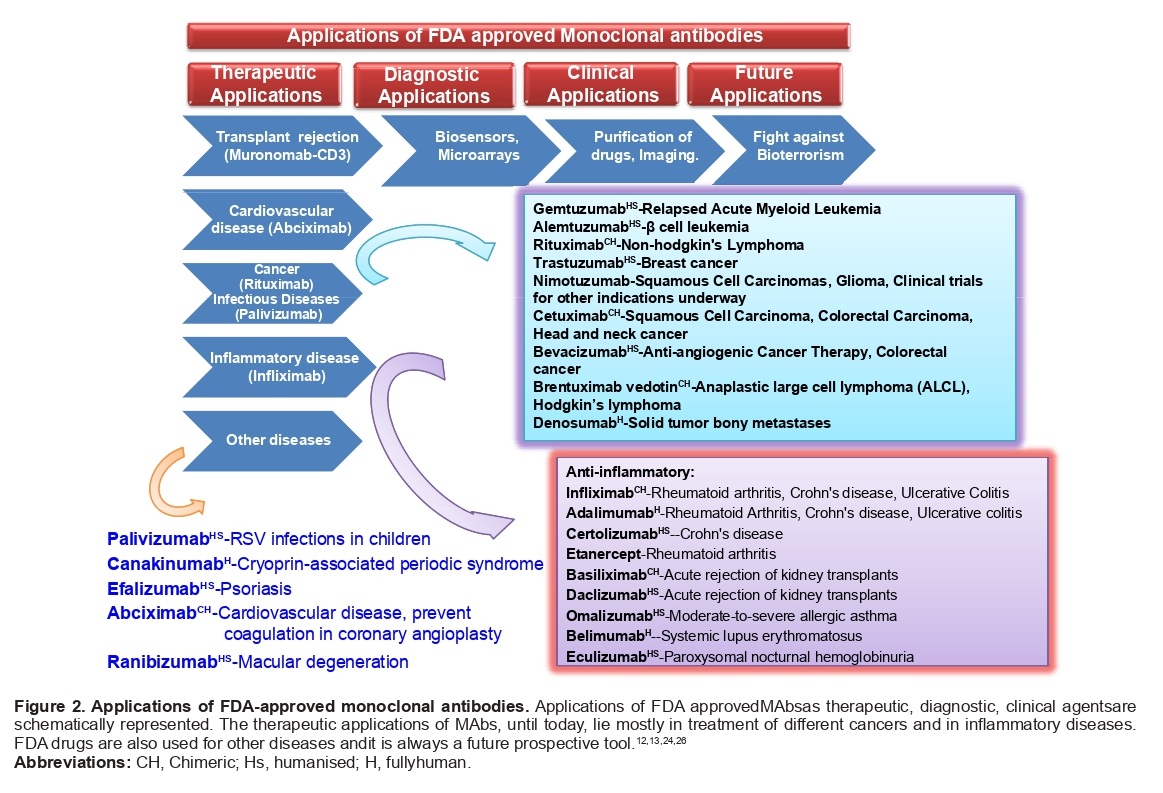
Applications and uses of MAbs
MAbs are highly specific and have proven to be valuable in various areas of research and applications. They are widely used in basic immunological and molecular research, commercial protein purification, diagnosis of diseases, suppressing the immune response, cancer therapy, identification of specialized cells, preparation of vaccines, and increasing the effectiveness of medical substances. Additionally, MAbs have applications in the diagnosis of allergies, hormone tests, purification of complex mixtures, and determining the structure of the cell membrane.
Advantages and Limitations of MABs
Compared to traditional therapies, monoclonal antibodies (mAbs) offer several advantages such as high specificity, potency, reduced toxicity, long half-life, low immunogenicity, potential for targeted delivery, and the potential for personalized medicine by targeting unique proteins or mutations in individual patients.
While mAbs have several advantages, there are also some limitations and potential side effects associated with their use.
Limitations:
Cost: mAbs are often expensive to produce and administer, making them less accessible to some patients.
Limited tissue penetration: mAbs are large molecules and may have difficulty penetrating some tissues or crossing the blood-brain barrier, limiting their effectiveness in treating certain conditions.
Development of resistance: Cancer cells can sometimes develop resistance to mAbs over time, reducing their effectiveness.
Development of neutralizing antibodies: Patients may develop antibodies against mAbs, reducing their effectiveness and potentially causing adverse reactions.
Potential side effects:
Infusion reactions: Some patients may experience allergic reactions or infusion-related reactions during or shortly after the infusion of the mAb.
Increased risk of infection: mAbs can suppress the immune system, increasing the risk of infections.
Development of autoimmune disorders: In rare cases, mAbs may trigger the development of autoimmune disorders.
Cytokine release syndrome: In some cases, mAbs can trigger an excessive release of cytokines, leading to flu-like symptoms and potentially more severe reactions.
Patients need to discuss the potential risks and benefits of mAbs with their healthcare provider before starting treatment.
Challenges and Opportunities for Further Development of Monoclonal Antibodies
Monoclonal antibodies have been successful in treating diseases, but challenges remain, including high production and treatment costs, resistance development, and immunogenicity. However, advancements in technology and biomanufacturing processes, combination therapies, and novel mAb formats offer opportunities for further development. Personalized mAbs and treatment of rare diseases are also important areas for future development.
References
- Mohammad Asaduzzaman Chowdhury, Nayem Hossain et.al; ”Immune response in COVID-19: A review”, Journal of Infection and Public Health, Volume 13, Issue 11, 2020, Pages 1619-1629, ISSN 1876-0341, https://doi.org/10.1016/j.jiph.2020.07.001.
- Charmsaz, Sara & Scott, Andrew. (2017). Targeted therapies in hematological malignancies using therapeutic monoclonal antibodies against Eph family receptors. Experimental Hematology. 54. 10.1016/j.exphem.2017.07.003.
- Voge NV, Alvarez E. Monoclonal Antibodies in Multiple Sclerosis: Present and Future. Biomedicines. 2019; 7(1):20. https://doi.org/10.3390/biomedicines7010020.
- Ansar W, Ghosh S. Monoclonal Antibodies: A Tool in Clinical Research. Indian Journal of Clinical Medicine. 2013; 4. doi:10.4137/IJCM.S11968.
- Batlevi CL, Matsuki E, Brentjens RJ, Younes A. Novel immunotherapies in lymphoid malignancies. Nat Rev Clin Oncol. 2016; 13:25–40.
- Specenier P, Vermorken JB.” Cetuximab: its unique place in head and neck cancer treatment”. Biologics. 2013; 7:77–90.



