Milstein and Köhler from Cambridge University were the pioneers in producing the first monoclonal antibodies (mAbs) which were initially made from murine proteins, making them immunogenic in humans and unsuitable for long-term therapy. However, with the use of molecular biology and protein engineering, more human-like mAbs with little immunogenicity were created. Different versions of mAbs and traps are being used in clinical trials and practice. Initially, the murine Fc sequences were replaced with human Fc in a process called “chimerisation”, where the murine antigen binding regions were grafted onto a human immunoglobulin backbone. In “humanisation”, the mouse hyper-variable peptide binding loops were grafted onto the human IgG backbone. Recently, newer technologies have enabled the creation of fully human antibodies.
Around a century ago, Paul Ehrlich put forward the concept of developing “magic bullets” that would specifically identify and treat affected cells, without harming healthy cells. Traditional therapies like chemotherapy and radiation, while effective at killing cancer cells, also damage healthy cells and limit treatment effectiveness. Monoclonal antibodies offer a potential solution by targeting cancer cells with high specificity, but there are challenges to their successful application. These challenges include low expression of target antigens, immune responses to the antibodies, and physical barriers preventing antibody binding.
Benefits of Monoclonal Antibody Therapy
Monoclonal antibody therapy has several benefits, including:
Targeted treatment: Monoclonal antibodies are engineered to specifically target certain cells or proteins, which makes them a precise treatment option with a lower risk of damaging healthy cells and tissues. This targeted approach also increases their effectiveness. The benefits of monoclonal antibody therapy include its targeted nature, which reduces the risk of side effects, and fast-acting response. Additionally, there is the potential for long-term immunity from the treatment.
Lower risk of side effects: Compared to conventional treatments such as chemotherapy or radiation therapy, monoclonal antibody therapy poses a lower risk of causing harmful side effects due to its highly specific mode of action.
Fast-acting: The therapeutic effects of monoclonal antibodies can be observed rapidly, typically within a few weeks of beginning treatment, due to their fast-acting nature.
Potential for long-term immunity: The use of monoclonal antibodies can lead to the activation of the immune system, which can result in long-lasting immunity to specific diseases like COVID-19. This immunity may persist beyond the duration of treatment and offer protection against future infections.
Some of the Successful Monoclonal Antibody Therapies
The efficacy and limitations of mAb in treating inflammatory diseases
The success of using anti-TNF therapy to treat rheumatoid arthritis (RA) was based on research showing that blocking TNF-α reduced the production of many other inflammatory cytokines in joints and cultures of rheumatoid synovium. However, the clinical response to anti-TNF therapy was heterogeneous, with some patients showing significant improvement while others did not. Genetic differences were initially considered as a possible reason for this, but recent clinical data has ruled it out. Although anti-TNF therapy is relatively safe, it does increase the risk of infections, particularly with intracellular organisms like tuberculosis. Nonetheless, anti-TNF therapy has been found to reduce complications and maintain a favorable benefit/risk ratio and is now used to treat a range of inflammatory diseases besides RA. Other monoclonal antibodies (mAbs) targeting cytokines like IL-6 and GM-CSF have also proven useful, as have ligand traps and CTLA4-Fc fusion proteins. However, some mAbs have been unsuccessful in treating certain conditions, such as rituximab in systemic lupus erythematosus (SLE).
Trastuzumab as an example of mAb Therapy in Solid Tumors
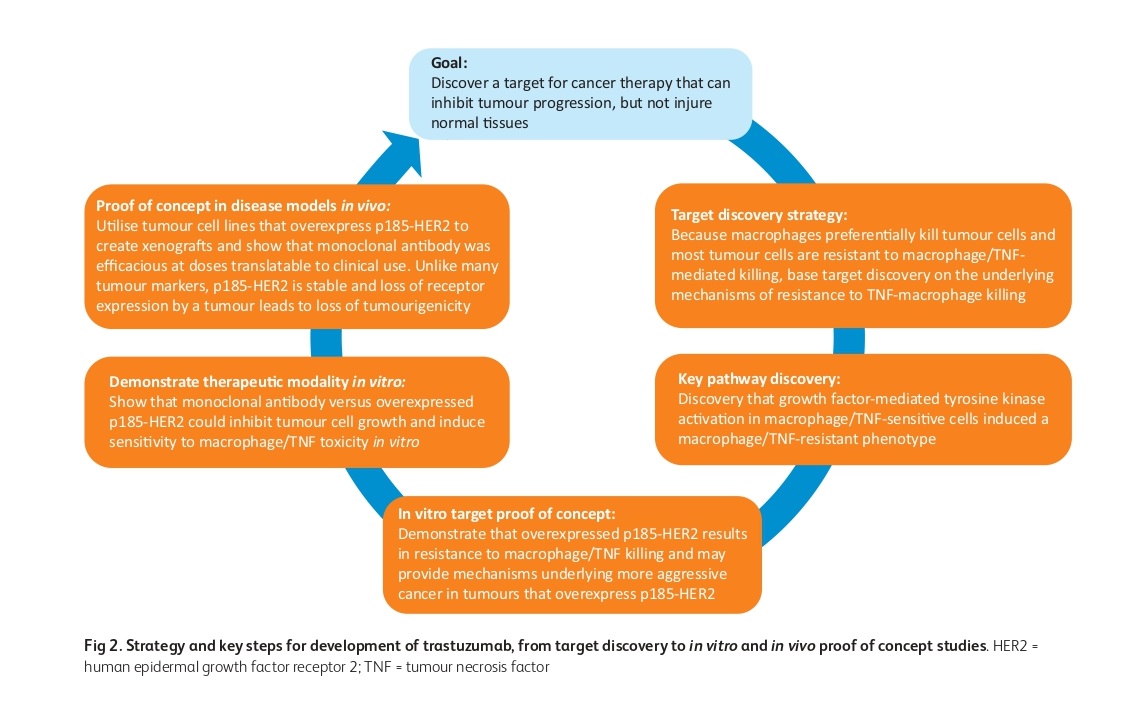
The development and success of mAb therapy in solid tumors, focusing on the example of trastuzumab were studied. The development of trastuzumab involved the identification of HER2 overexpression in breast and ovarian cancers and the discovery that overexpression of HER2 induces resistance to TNF, a growth inhibitor. The lead antibody, MuMAb4D5, was humanized using a consensus human variable domain sequence and has three mechanisms of action dependent upon upregulated HER2: growth inhibition, antibody-dependent cell-mediated cytotoxicity (ADCC), and induction of TNF sensitivity. Trastuzumab was successful in treating HER2-overexpressing tumors, which validated the importance of the stability of the target itself and led to the development of other mAbs, such as pertuzumab and trastuzumab emtansine, which bind to separate epitopes on HER2. The success of trastuzumab required the co-development of two novel companion diagnostic approaches: immunohistochemistry and fluorescence in situ hybridization for selecting patients most likely to respond.
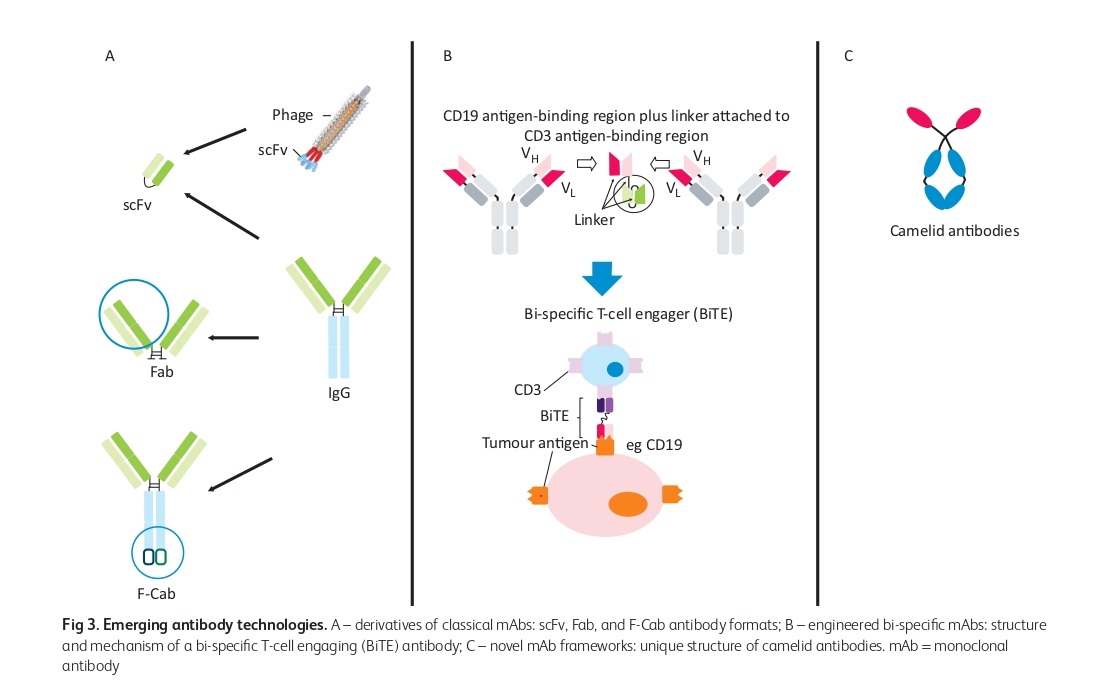
Bi-specific mAbs and their use in catumaxomab for malignant ascites
The use of Fab fragments in ranibizumab (Lucentis) can be facilitated by manufacturing them in Escherichia coli. However, the short half-life of Fabs can be overcome by polyethylene glycol, as demonstrated in certilizumab pegol. In the past, the synthesis of bi-specific mAbs was challenging. However, there are now various types of bi-specific mAbs available. Bi-specific T-cell engaging (BiTE) antibodies are composed solely of variable regions from distinct antibodies. The first approved bi-specific antibody, catumaxomab, for malignant ascites, is in this format.
Limitations of Monoclonal Antibody Therapy
Monoclonal antibody therapy has several limitations, including limited availability and accessibility, high cost, the need for early intervention, potential resistance and mutation, and the risk of side effects. These factors may make it difficult for some patients to receive the treatment or achieve optimal results. Close monitoring and management of side effects are necessary for patients receiving monoclonal antibody therapy.
Challenges and Considerations in the Production of Monoclonal Antibodies
Various techniques utilizing hybridoma technology are employed for producing monoclonal antibodies using bacterial, yeast, and mammalian cell lines, as well as transgenic animals. The biological materials used in these methods need to be free from microbial contamination by viruses, bacteria, fungi, mycoplasma, and prions. Animals must be specific pathogen-free (SPF) quality. At present, the primary means of commercially producing antibodies is through recombinant expression technology in mammalian cell culture. The use of human mAbs produced in cell lines that have been immortalized through the introduction of Epstein-Barr virus (EBV) may present a risk of cancer. Additionally, there are other concerns, such as the risk of contamination of different cell lines, production of endogenous immunoglobulin chains, genetic instability of the cell line, and consistency of the expression of both heavy and light chains. During mAb production, it is also challenging to generate equal amounts of both heavy and light chains in the production cell lines, as imbalances in chain production can be toxic to cells or can result in non-functional immunoglobulin chains in the supernatant, making the purification process more complicated.
Side Effects of mABs
Monoclonal antibodies can cause side effects, similar to other therapeutic agents. These side effects can be related to three primary mechanisms: the xenogenic nature of the antibodies, suppression of physiological functions related to the specificity of the antibody, and activation of inflammatory cells and mediators after binding to the target. Compared to cytotoxic chemotherapeutic agents used in cancer treatment, monoclonal antibodies are generally considered less toxic. Mechanism-independent toxicities can occur due to hypersensitivity reactions, which can rarely be severe enough to cause anaphylactic reactions. Mechanism-dependent toxicities may include cardiac toxicity with trastuzumab, first-dose toxicity related to rapid lysis of malignant cells by rituximab, skin eruptions with cetuximab, and hypertension, bleeding, thrombosis, and proteinuria with bevacizumab. While hypersensitivity reactions leading to serum sickness are generally rare, some mAbs have been associated with fatal infusion reactions.
The Role of FcRn in the Serum Half-Life of mAbs
MAbs designed to target tumor-specific antigens often remain in the bloodstream and interact with the tumor cells inefficiently in murine xenograft models. The ability of mAbs to penetrate and remain in targeted tissue depends on their molecular characteristics such as size, shape, affinity, and valency. Due to their large size, mAbs have long serum half-lives and cannot be eliminated through the kidney glomeruli. Furthermore, the Fc portion of IgG molecules can interact with various receptors, including the neonatal Fc receptor (FcRn), which is expressed in several cell types, including vascular endothelial cells, monocytes, macrophages, and barrier sites such as the blood-brain interface, the glomerular filter in the kidneys, and the intestinal epithelium. FcRn plays a crucial role in IgG homeostasis and extends the serum half-life of IgG and albumin by shutting IgG molecules away from lysosomes and back into the serum. This protective carrier confers on them an even longer serum half-life.
Limitations of ADCC Activation Through Therapeutic Antibodies
The activation of ADCC through therapeutic antibodies is subject to various limitations. Firstly, the affinity between the Fc and its receptors is critical, but 80% of the population expresses a low-affinity variant of the receptor, which poses a significant challenge. Secondly, the glycosylation of IgG1 molecules in the CH2 domain of the Fc region is essential as it modulates the Fc’s affinity for FcγRIIIa and affects the in vivo efficacy of antibodies. The presence of fucose residues in the carbohydrate decreases ADCC efficiency, and the type of carbohydrate depends on the cell line used for antibody production. For instance, an anti-CD20 chimeric IgG1 produced by the rat hybridoma YB2/0 cell line has over 50-fold higher ADCC than the same antibody produced by the Chinese hamster ovary (CHO) cell line, which is typically used for therapeutic protein production. This difference is attributed to the high expression of FUT8, which is responsible for α1,6-fucosyltransferase, in the CHO line.
References
- Jones PT, Dear PH, Foote J, Neuberger MS, Winter G., “Replacing the complementarity-determining regions in a human antibody with those from a mouse”, Nature 1986; 321: 522 – 5.
- Slamon DJ, Clark GM, Wong SG, et al.,” Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neuoncogene”, Science 1987; 235: 177 – 82.
- Ferrara N, Damico L, Shams N, Lowman H, Kim R., “Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration”. Retina 2006; 26: 859 – 70.
- Mahoney KM, Rennert PD, Freeman GJ., “Combinatino cancer immunotherapy and new immunomodulatory targets”, Nat Rev Drug Discov 2015; 14: 561 – 84.
- Shepard HM, Phillips GL, D Thanos C, Feldmann M., “Developments in therapy with monoclonal antibodies and related proteins”, Clin Med (Lond). 2017 Jun; 17(3):220-232. Doi: 10.7861/clinmedicine.17-3-220. PMID: 28572223; PMCID: PMC6297577.
- Chames P, Van Regenmortel M, Weiss E, Baty D.,” Therapeutic antibodies: successes, limitations, and hopes for the future”, Br J Pharmacol. 2009 May; 157(2):220-33. Doi: 10.1111/j.1476-5381.2009.00190.x. PMID: 19459844; PMCID: PMC2697811.