The Clinical Laboratory Improvement Amendments (CLIA) establishes quality standards for all laboratory testing to ensure accurate and reliable results. Meeting these standards can be challenging for laboratories, as they require compliance with regulations regarding personnel qualifications, quality control, proficiency testing, and record-keeping. Laboratories must also meet specific standards for each category of testing they perform, such as microbiology, hematology, or chemistry. Ensuring compliance with these regulations requires significant resources, including personnel, equipment, and time. Furthermore, new technologies and testing methods constantly emerge, presenting both opportunities and challenges for laboratories seeking to stay up-to-date and meet CLIA standards. However, meeting these standards is crucial to providing accurate and reliable laboratory testing and ensuring the safety and well-being of patients.
Liability Risks and Error Rates in Clinical Laboratory Testing
Clinical laboratories can be held liable for medical malpractice either directly or indirectly. Errors in the testing process can occur during the pre-analytic, analytic, and post-analytic phases, or a combination of these phases. Studies have shown that pre-analytic factors account for a higher percentage of errors (46-68.2% of total errors), but a significant number of errors also occur in the post-analytic phase (18.5-47% of total errors). In the pre-analytic phase, errors such as incorrect test ordering are a major cause of missed or delayed diagnoses in both ambulatory and emergency department settings. Non-analytic errors, such as errors in test ordering, implementation, result reporting, clinician responses, patient notification, and communication, are often caused by system dysfunctions, and account for a significant percentage of errors. Charting or filing errors are another common cause of errors, accounting for 14.5% of all errors.
While the incidence of errors caused by analytical factors has decreased over time, some processes like immunoassays still carry a risk of errors. However, it is primarily the analytical phase of clinical laboratory testing that falls under the direct control of the laboratory. On the other hand, the pre-and post-analytical phases are more likely to be the responsibility of healthcare professionals such as clinicians, nurses, and those involved in specimen acquisition, patient identification, labeling, data entry, specimen collection, and transport.
Clinical Laboratory Liability and Critical Laboratory Results Communication
Laboratory errors can have a significant impact on patient care, as over 70% of medical decisions are based on laboratory data. Erroneous laboratory results can compromise diagnosis and treatment decisions, leading to potential harm to patients. Delayed or incorrect laboratory results can result in joint liability for providers, laboratories, and institutions in medico-legal actions alleging delay in diagnosis, failure to diagnose, or erroneous diagnosis. In 2008, the International Organization for Standardization recognized that laboratory errors can cause a failure of planned action, occurring at any part of the laboratory cycle, from ordering examinations to reporting results and appropriately interpreting and reacting to them, and published Technical Specification (ISO/TS 22367) to address this issue.
Challenges and Concerns Related to Genetic Testing and Confidentiality
Medical science has made significant progress in identifying genetic links between genotype and disease states, with genetic tests now available for thousands of diseases. The FDA is responsible for overseeing laboratory tests and test kits but only regulates under CLIA and not test development. As a result, there are concerns about protecting research participants during test development, the validity of data generated by genetic tests, the confidentiality of genetic information, and the validity and confidentiality of genetic tests marketed directly to consumers. For example, if insurers and employers learn of a research participant’s positive test results for biomarkers of Alzheimer’s disease pathology, the individual may be at risk for discrimination. Certificates of confidentiality only provide limited protection as they only prohibit researchers from disclosing identifiable sensitive information gathered during research.
Regulatory and Business Responsibilities of Laboratory Directors and Compliance Officers
According to federal law, laboratory directors are responsible for the overall administration, operation, and employee competence of laboratories. In some healthcare organizations, compliance officers may also oversee adherence to regulations. In 1997, the US Department of Health and Human Services (HHS) Office of the Inspector General (OIG) developed a model compliance plan for laboratories to follow regarding marketing and billing practices. This plan included the responsibilities of individuals monitoring billing practices for payment from federal programs. However, in practice, these responsibilities have expanded to include compliance with all billing practices and regulations. Laboratory directors are responsible for ensuring both the quality of testing and appropriate business practices. The importance of the laboratory director is highlighted by the fact that directorship was involved in two of the top 10 serious condition-level deficiencies identified by the Centers for Medicare and Medicaid Services (CMS) in 2018.
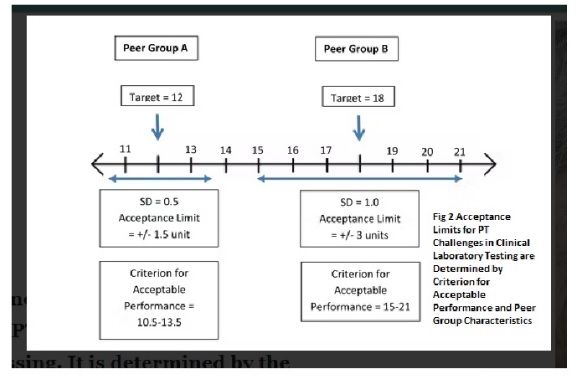
Establishing Target Values and Grading for Quantitative Analytes in Clinical Laboratory Testing
In clinical laboratory testing, quantitative analytes are those that are reported in numerical values, such as blood glucose levels or cholesterol levels. To ensure accurate and reliable results, a target value is established for each quantitative analytes. There are two main ways to establish the target value for a quantitative analytes. One way is to use the overall average of participant responses, after removing any outliers. Another way is to establish the target value based on the results from a minimum of ten referee laboratories. While the idea of using definitive or reference methods to establish targets was established by CLIA, it is not always possible to do so. Therefore, it is uncommon to set the target value using a reference method. If there is not at least 80% agreement among results for a quantitative analytes, it is not graded. This means that at least 80% of results must fall within the criteria for acceptable performance as specified in Subpart I of the CLIA regulations. This ensures that laboratories are meeting minimum standards for accuracy and reliability in their testing.
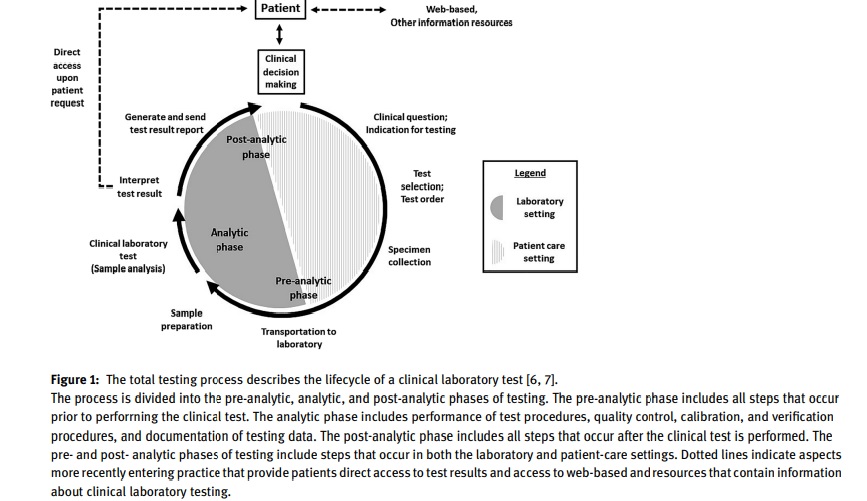
The Intersection of Diagnostic Excellence and Laboratory Practice through the Total Testing Process (TTP)
Diagnostic excellence is a system-level state that integrates healthcare knowledge, skills, and resources to continuously improve diagnoses and reduce the risk of diagnostic errors while meeting the needs of patients and healthcare systems. The concept of diagnostic excellence intersects with laboratory practice through the total testing process (TTP), which was first described by Lundberg in 1981 and defined by the US Centers for Disease Control and Prevention (CDC) in 1986 and revisited in 2011. Lundberg emphasized the need for continuous assessment of the laboratory test’s effects and performance on patients and public health. A 2008 CDC report advocated for enhanced engagement between clinical laboratories and other healthcare providers to improve health outcomes. Epner et al. revisited this concept in 2013, advocating for an outcomes-based approach that links laboratory processes to accurate and timely diagnoses to support diagnostic excellence. However, at the time, there were limited studies linking TTP elements to accurate and timely diagnoses and measurable health outcomes.
Opportunities in Meeting CLIA Standards for Laboratory Testing
Meeting the Clinical Laboratory Improvement Amendments (CLIA) standards is essential for any laboratory that performs testing on human specimens. Not only is compliance with these standards required by law, but it also ensures the accuracy and reliability of laboratory test results, which is critical to patient care.
Here are some opportunities that come with meeting CLIA standards for laboratory testing:
Improved Patient Outcomes: CLIA standards require laboratories to follow strict quality control and quality assurance procedures. Meeting these standards ensures that test results are accurate and reliable, leading to improved patient outcomes. Accurate test results can help healthcare providers make informed decisions about diagnosis and treatment, leading to better patient outcomes.
Increased Patient Satisfaction: Patients are more likely to trust and have confidence in a laboratory that meets CLIA standards. Meeting these standards can help to increase patient satisfaction and confidence in the laboratory’s ability to perform testing accurately and reliably.
Competitive Advantage: Meeting CLIA standards can be a competitive advantage for laboratories. Laboratories that comply with these standards can differentiate themselves from those that do not, and this can help to attract new clients and customers.
Compliance with Legal and Regulatory Requirements: CLIA standards are mandated by law, and failure to comply can result in penalties and other legal consequences. Meeting these standards ensures compliance with legal and regulatory requirements, which can help to avoid legal issues and maintain a good reputation.
Opportunities for Growth: Laboratories that meet CLIA standards can expand their services to include new types of testing or offer to test in new geographic areas. This can help to grow the business and increase revenue.
References
- Kachalia A, Gandhi TK, Puopolo AL, et al. Missed and delayed diagnoses in the emergency department: a study of closed malpractice claims from 4 liability insurers. Ann Emerg Med. 2007;49(2):196–205. https://doi.org/10.1016/j.annemergmed.2006.06.035.
- Notice of changes to NIH policy for issuing certificates of confidentiality. Notice Number: NOT-OD-17-109. September 7, 2017. Available online at: https://grants.nih.gov/grants/guide/ notice-files/ NOT-OD-17- 109.html…
- Lundberg G. Critical (panic) value notification: an established laboratory practice policy (parameter). JAMA. 1990; 263:709.
- Lippi G, Mattiuzzi C. Critical laboratory values communication: summary recommendations from available guidelines. Ann Transl Med. 2016;4(20):400. https://doi.org/10.21037/2016.09.36.
- Goldenstein R, Prosser D, Travis L. Analysis of critical laboratory value nurse-to-physician follow-up calls at a community hospital. J Nurs Care Qual. 2013; 28(4):335–9.https://doi.org/10.1097/NCQ.0b013e31829229ff.
- Top 10 Conditions in the Nation _ CMS Surveys (2018). , https:// cms.gov/Regulations-and-Guidance/Legislation/CLIA/.
- Lubin, Ira M., Astles, J. Rex, Shahangian, Shahram, Madison, Bereneice, Parry, Ritchard, Schmidt, Robert L. and Rubinstein, Matthew L… “Bringing the clinical laboratory into the strategy to advance diagnostic excellence” Diagnosis, vol. 8, no. 3, 2021, pp. 281-294. https://doi.org/10.1515/dx-2020-0119



