In 1890, Behring and colleagues used antibodies from immunized animals to cure non-immunized animals infected with diphtheria and tetanus. This led to the development of g-globulin therapy. Mouse hybridoma technology was introduced in 1975, allowing the production of identical antibodies. However, mouse monoclonal antibodies had limitations as human therapeutics due to their inability to trigger human effectors’ functions and immune recognition leading to human anti-mouse antibodies. Despite this, mouse monoclonal antibodies were successful in preventing organ graft rejection. Incidence of immunogenicity varied based on the target antigen, the disease being treated, and the administration schedule, with solid tumor patients having higher rates of human anti-mouse antibodies than those with relapsed B-cell malignancies.
Importance of Monoclonal Antibodies
Monoclonal antibodies (mAbs) are laboratory-created molecules that mimic the immune system’s attack on cancer cells by binding to antigens on their surface. This concept dates back to the 18th century when it was discovered that fluid from a smallpox pustule provided immunity. The first mAbs were developed in the 1930s and 1940s, and the technique of creating them involved immunizing experimental animals. The first fully licensed mAb for human use was developed in 1986, and the technology has since been improved to create fully humanized antibodies. The first mAb approved by the FDA was muromonab-CD3, which was used as an anti-rejection medication. The first successful mAb in treating solid tumors was trastuzumab, which targeted HER2 biomarkers. The development of trastuzumab led to the discovery of biomarker-targeted cancer treatments.
Development of Monoclonal Antibodies
Hybridoma technology
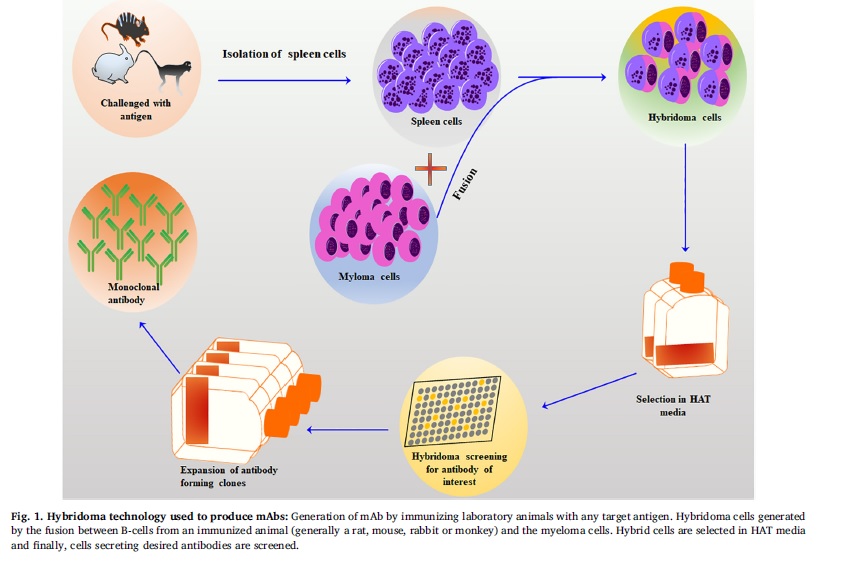
The hybridoma technology involves the fusion of an antibody-producing B cell with an immortal myeloma cell to produce a stable cell line that secretes a large number of specific mAbs. The technology was initially limited to murine antigens but has been extended to different species, including humans, rabbits, chickens, goats, sheep, cows, mice, guinea pigs, and rats. The choice of animal species for mAb isolation depends on several factors, including the availability of a suitable fusion partner and the purpose for which the mAbs are needed. The mice hybridoma technology is a multi-step process that involves the development and optimization of specific immunogenic antigens, immunization of host animals, selection of animals with high titers of binding antibodies, isolation of splenocytes, fusion with immortalized myeloma cells, and screening of hybrid cells by “limited dilution cloning” method shown in fig 1.
Phage display technology
The phage-display system is a powerful technique that involves cloning millions of variants of certain ligands (such as peptides, proteins, or fragments) into the phage genome as a fusion to one of the phage coat proteins. This results in the presentation of the ligand on the phage surface and allows for the enrichment of specific phage by selecting those that bind to an immobilized target. The genetic material of the ligand resides within the phage particle, allowing for the connection between genotype and phenotype. After recovery of bound phage, they can be replicated to enrich those clones recovered from the library and further analyzed. The success of this technique relies on the synthesis of large combinatorial repertoires on phage and the combination of display and enrichment.
Transgenic Mice Technology
Transgenic mice technology is commonly used to develop monoclonal antibodies for diseases like cancer, autoimmune disorders, and infectious diseases. The process involves inserting the gene encoding the target antigen into the mouse genome and immunizing the mice to stimulate antibody production. The antibody-producing cells are fused with myeloma cells to create hybridoma cells that produce large quantities of monoclonal antibodies. These cells are screened and selected for producing desired mAbs, grown in large culture volumes, and then purified. This technology has transformed mAb development and improved the lives of millions of patients.
Humanization Technology
To humanize a monoclonal antibody, the procedure involves identifying a mouse monoclonal antibody that targets a specific protein, isolating the genetic sequence coding for the antibody’s variable regions, using recombinant DNA technology to replace mouse variable regions with human ones, and testing the resulting hybrid antibody for its ability to bind to the target protein. Additional genetic engineering is done to remove mouse sequences and create a fully-humanized monoclonal antibody. The safety and efficacy of the humanized antibody are evaluated in preclinical and clinical trials, and regulatory approval is obtained if found safe and effective as a therapeutic agent. Humanization helps create monoclonal antibodies more similar to human antibodies, minimizing risks and enhancing efficacy in treating human diseases. The development of monoclonal antibodies, structure and function are shown in fig.2.
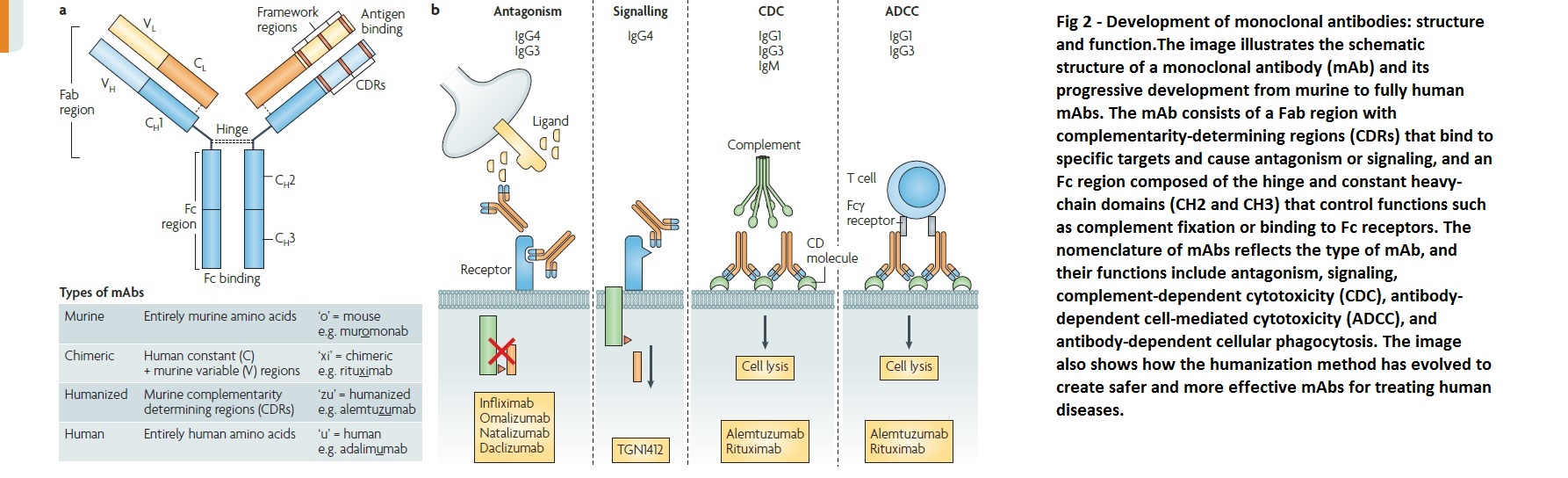
Types of Monoclonal Antibodies
The four categories of monoclonal antibodies are murine, chimeric, humanized, and human.
Murine monoclonal antibodies
The first monoclonal antibody (mAb) discovered and reproduced was murine, derived from mouse spleenic B lymphocytes fused with an immortal myeloma cell line lacking the HPTR gene. All murine mAbs end in -omab and are prone to induce allergic reactions and the production of anti-drug antibodies in humans. Due to weak binding to human FcRn, they also have a short half-life in humans. In oncology, murine mAbs may not be the most effective as they are relatively poor at recruiting essential functions such as antibody-dependent cellular cytotoxicity and complement-dependent cytotoxicity needed for tumor destruction.
Chimeric monoclonal antibodies
Chimeric mAbs are created by utilizing the murine antigen-specific variable region, while the remaining heavy and light chains are human, resulting in mAbs that are approximately 65% human and 35% murine, achieved through genetic engineering techniques. These mAbs are identified by names ending in -ximab and have an extended half-life in humans and reduced immunogenicity compared to their murine counterparts. However, they still have a considerable propensity to induce anti-drug antibodies.
Humanized monoclonal antibodies
Humanized mAbs are produced by grafting the murine hyper variable regions onto a human antibody framework, resulting in molecules that are about 95% human. This approach leads to decreased production of anti-drug antibodies, although it is a difficult and limited process. These mAbs are identified by names ending in -zumab, such as trastuzumab, alemtuzumab, and bevacizumab.
Fully human monoclonal antibodies
New technology has enabled the creation of fully human mAbs using animals carrying human Ig genes. These transgenes contain parts of the variable regions, allowing for the recombination of human antibodies. The animal’s endogenous Ig genes are inactivated, enabling the generation of fully human mAbs. These mAbs are less antigenic and better tolerated compared to other classes of mAbs. Additionally, they seem to remain present in the human body’s circulation compared to other classes. Fully human mAbs are identified by names ending in -umab, such as ofatumumab, daratumumab, and denosumab.
Classification of Monoclonal Antibodies (mAbs) based on Administration and Use
Monoclonal antibodies (mAbs) can be classified into three types based on their administration or use: unconjugated/naked, conjugated, and bispecific. Unconjugated mAbs function independently by attaching to antigens on cancer cells, leading to enhanced immune response and recognition of cancer cells by the immune system, ultimately resulting in increased apoptosis. Another mechanism of unconjugated mAbs is blocking antigens on cancer cells that aid in their proliferation. Conjugated mAbs are those combined with chemotherapy agents or radioactive particles, serving as a delivery mechanism for these agents to minimize harm to normal cells. Bispecific mAbs combine two different mAbs to attach to two different antigens simultaneously, one on cancer cells and one on immune cells, in the hopes of inciting an increased immune response and destruction of cancer cells.
Applications of Monoclonal Antibodies
Therapeutic Applications
Historically, pharmacology relied on synthetic chemistry and natural sources, but now protein-based therapeutics, such as monoclonal antibodies (mAbs), are prevalent thanks to advancements in molecular biology and immunology. Unlike polyclonal antibodies (pAbs), which are heterogeneous and unsuitable for therapeutic purposes, mAbs are mono-specific and homogeneous. Hybridoma technology enabled the selection of high-affinity mAbs, but the murine origin of the first mAb approved for human use, muromonab-CD3, posed limitations. However, genetic engineering has since allowed for the development of chimeric, humanized, and fully human mAbs. Today, there are approximately 80 mAbs with marketing approval, and their targets have expanded to include membrane-bound targets, necessitating pharmacological characterization.
Cancer Therapy
Monoclonal antibodies (mAbs) have been developed to treat different types of cancers, both blood-related and solid tumors. These mAbs target specific tumor antigens, such as growth factor receptors that are over-expressed in cancer cells. By blocking these receptors, the mAbs can prevent ligand binding and signaling, leading to a decrease in tumor growth, apoptosis, and increased sensitivity to chemotherapy. For example, trastuzumab (Herceptin) is a mAb that targets HER2, a receptor that is over expressed in 30% of invasive breast cancers and inhibits receptor dimerization and internalization. Other targets for mAbs include hematopoietic differentiation antigens found on the surface of normal and tumor cells. Rituximab (Rituxan), a mAb used for lymph proliferative disorders, targets CD20, a pan B-cell marker, and promotes interactions between immune cells and the mAb’s Fc region.
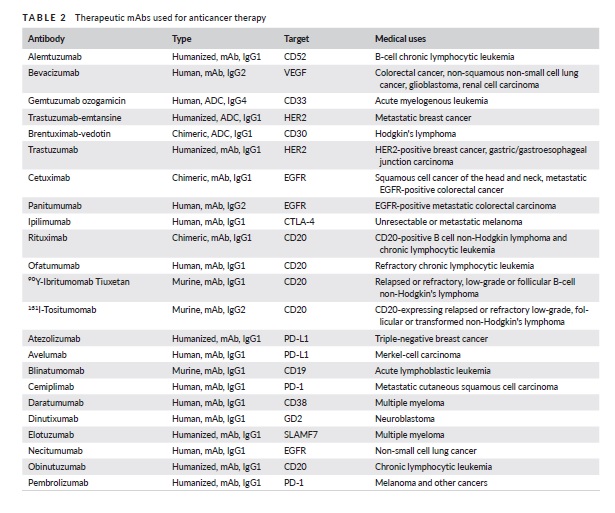
Autoimmune Disease Therapy
Monoclonal antibodies have revolutionized the treatment of autoimmune diseases by targeting different components of the immune system. Autoimmune diseases are caused by the activation of auto-reactive CD4+ lymphocytes that migrate to the targeted organ and cause damage. MAbs can block and deplete T and B cells, inhibit their interaction, block recruitment, and differentiation or activation, as well as pro-inflammatory cytokines. TNF-α is the most common cytokine targeted, which induces inflammation and is essential in autoimmunity. Antibodies targeting TNF-α have been effective in treating rheumatoid arthritis, psoriatic arthritis, and Crohn’s disease.
Immunoassays
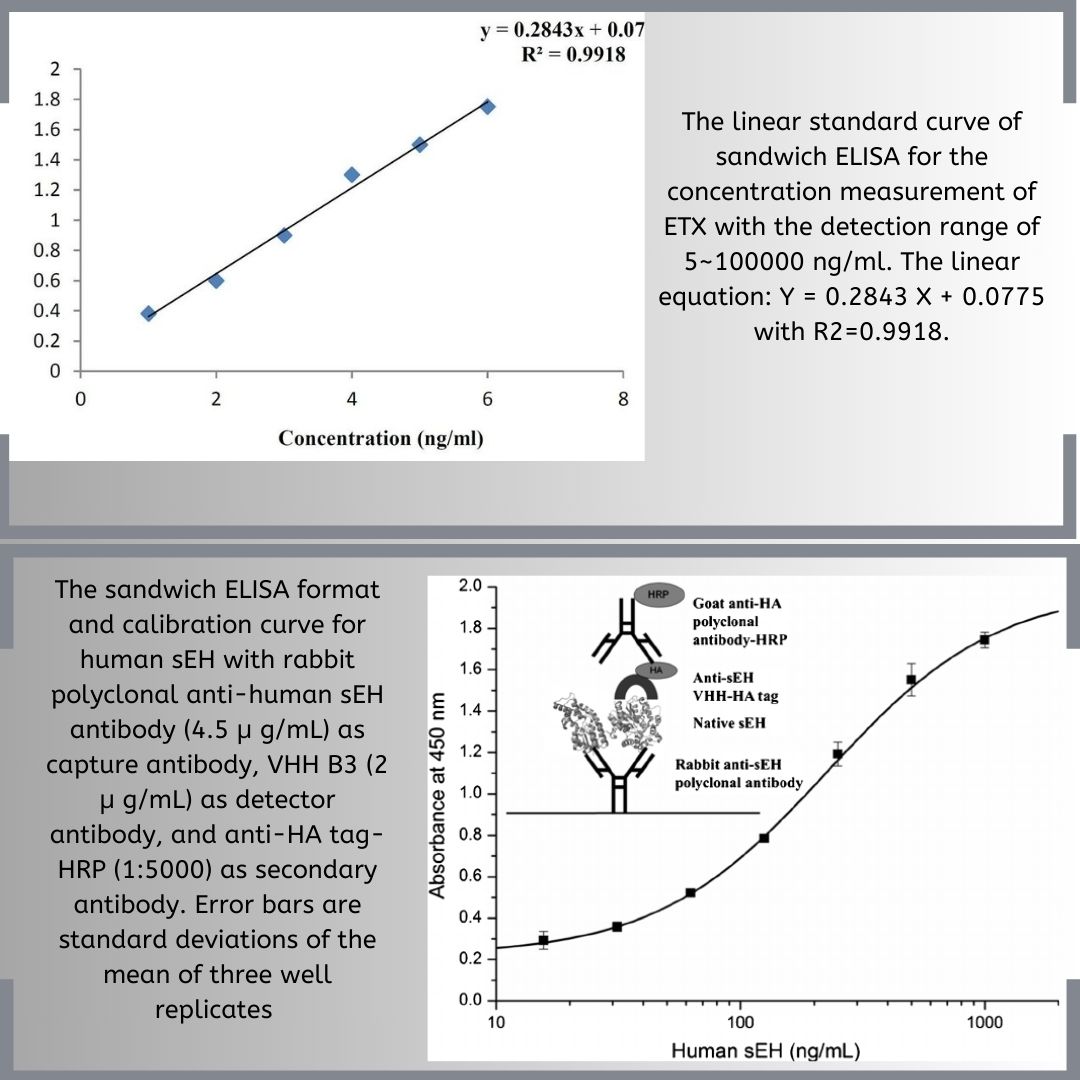
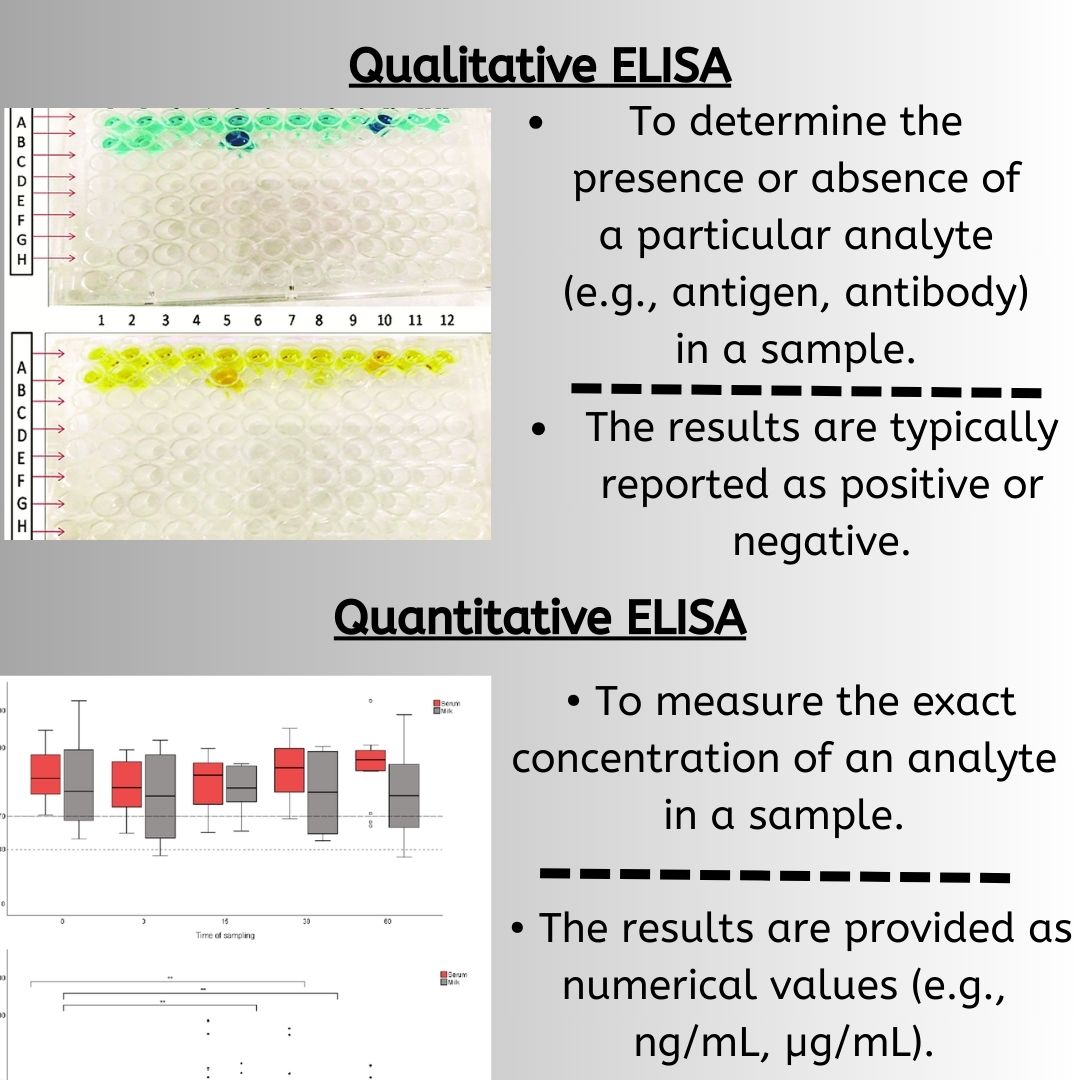
Immunoassays are essential tools for monoclonal antibody development and characterization, relying on the specificity of mAbs to detect and quantify target analytes. ELISAs, RIAs, and FIAs are common immunoassays used for screening hybridoma cell lines, quantifying antibody concentrations, and evaluating binding properties. These assays aid in identifying clones producing monoclonal antibodies with desired specificity and affinity, monitor changes in binding activity during development and manufacturing, and quantify the final drug product. Immunoassays thus have a crucial role in the development and characterization of monoclonal antibodies.
Immunohistochemistry
Immunohistochemistry is a technique that uses monoclonal antibodies to detect specific proteins in tissue samples. In this technique, a tissue sample is treated with the monoclonal antibody, which binds to the target protein if present. The bound antibody is then detected by a secondary antibody that is conjugated to a reporter molecule, such as an enzyme or fluorescent dye, allowing visualization of the protein’s location and distribution within the tissue sample. This technique is widely used in diagnostic and research settings to identify specific proteins and biomarkers in tissue samples, which can aid in disease diagnosis, prognosis, and treatment planning.
Challenges in Monoclonal Antibody Development
Production: To ensure quality, biological materials and animals used in monoclonal antibody (mAb) production must be free of microbial contamination and specific pathogen-free (SPF). Consistency in mAb production is critical, as minor changes in the production process can affect antibody specificity. Mammalian cell culture-based production systems are expensive and time-consuming and have limited control over mAb glycosylation, which affects stability and biological activity. Production capacity in these systems may become a limiting factor shortly. Transgenic animals may offer a cost-effective solution for extended high-dose mAb production.
Cost: Setting up a large-scale monoclonal antibody production system can be very expensive, costing approximately 100 million Euros initially with additional investments ranging from 300-500 million Euros. The success of the production system depends on the intrinsic characteristics of hybridomas, including cell growth and antibody production ability. The high cost of monoclonal antibody therapy, which can be up to $10,000 per month and $100,000 per year per patient, is a significant challenge.
Mechanism-Dependent Toxicities and Hypersensitivity Reactions Associated with Monoclonal Antibodies
The administration of monoclonal antibodies (mAbs) in large doses can lead to various issues such as high cost, administration challenges, and increased immunogenicity. The use of infusion-based treatments may result in varying levels of bioavailability and an increased probability of immune response. The development of humanized and fully human mAbs has reduced immunogenicity rates to 5-10%, but they can still cause side effects related to their nature as xenogeneic molecules, suppression of physiological functions, and activation of inflammatory cells and mediators. Although mAbs are generally less toxic than cytotoxic chemotherapeutic agents, they can cause hypersensitivity reactions, including anaphylactic reactions. Mechanism-dependent toxicities have also been reported with certain mAbs, such as cardiac toxicity with trastuzumab, first-dose toxicity related to rapid lysis of malignant cells by rituximab, skin eruptions with cetuximab, and hypertension, bleeding, thrombosis, and proteinuria with bevacizumab. Fatal infusion reactions have been reported with some mAbs, although hypersensitivity reactions leading to serum sickness are generally rare.
Potential Improvements of Future Antibody Therapies
To address the limitations of current monoclonal antibody (mAb) treatments, gene transfer technology has been explored as a potential solution. This involves delivering therapeutic mAb genes directly into the patient’s cells, which can continuously and stably produce and release low but effective amounts of mAbs. This approach offers the advantage of potentially reducing immunogenicity and improving the bioavailability of the therapeutic proteins while minimizing side effects and immune responses caused by high-dose infusion-based treatments. There are three main approaches to achieving long-term expression of therapeutic antibodies in vivo: direct in vivo administration of gene-carrying vectors, grafting of genetically modified autologous cells, and implantation of transduced cells encapsulated within membranes. However, regulating the production of therapeutic antibodies is crucial to avoid undesired side effects or if the therapy is no longer needed. This can be achieved using different transcriptional switches, inducible systems, or incorporating a suicide gene, such as herpes simplex virus thymidine kinase, in the transgene cassette.
References
- PandeyM, MahadevanD.”Monoclonal antibodies as therapeutics in human malignancies”, FutureOncol.2014; 10: 609–636.
- KaunitzJ, “Development of monoclonal antibodies: the dawn of mAbrule”, DigDisSci.2017; 62: 831–832.
- ShepardHM, PhillipsGL, et.al; “Developments in therapy with monoclonal antibodies and related proteins”, ClinMed (Lond).2017; 17: 220–232.
- BussNA, HendersonSJ, et.al; “Monoclonal antibody therapeutics: History and future”, CurrOpinPharmacol.2012; 12:615–622.
- Iannello A, Ahmad alanine, “Role of antibody-dependent cell-mediated cytotoxicity in the efficacy of therapeutic anticancer monoclonal antibodies”, Cancer Metastasis Rev. 2005; 24:487_99.
- Sethuraman N, Stadheim TA., “Challenges in therapeutic glycoprotein production. Curr Opin Biotechnology”, 2006; 17: 341_6.
- Berenson A.” A cancer drug shows promise, at a price that many can’t pay”, New York Times. 2006 Jun 15.