Antibody-Drug Conjugates (ADCs) are drugs consisting of a tumor-specific monoclonal antibody (mAb) linked to a potent cytotoxin via a stable linker. They are capable of delivering cytotoxins directly to cancer cells, reducing systemic exposure and increasing drug potency, resulting in fewer side effects and a wider therapeutic window. The first generation of ADCs failed to significantly improve drug specificity and reduce toxicity, but internalizing conjugates have increased their therapeutic potential. Three ADCs have gained entry into the market, with only two remaining, brentuximab vedotin and trastuzumab emtansine, approved for use against Hodgkin’s lymphoma, anaplastic large cell lymphoma, and breast cancer.
Overview of the Development of ADCs
Creating a Successful ADC and its Mechanism of Action
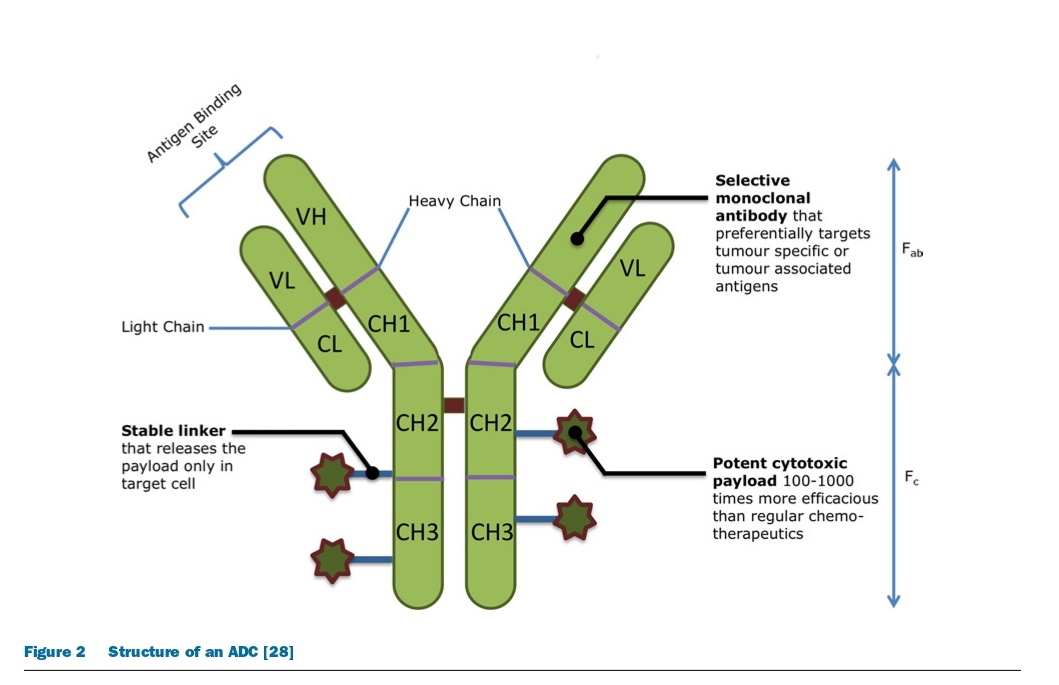
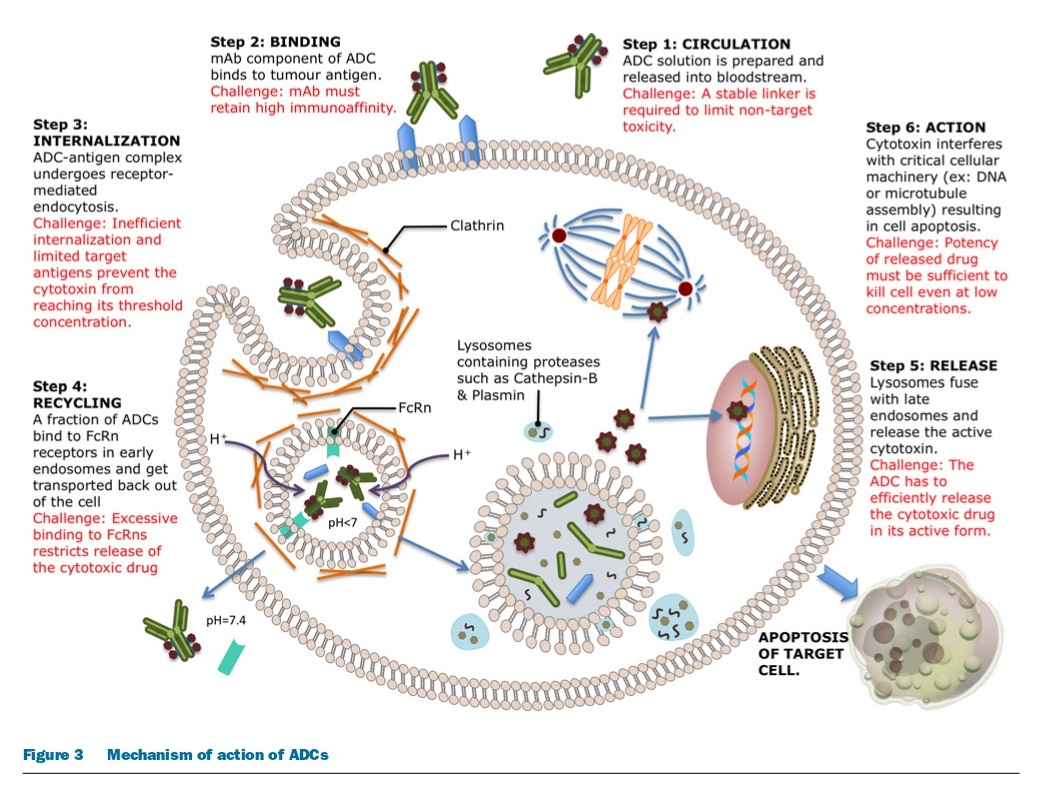
To design a successful ADC, it is important to understand its mechanism of action and determine the necessary characteristics of all three components: the monoclonal antibody, linker, and cytotoxic drug. The ideal ADC should retain the specificity and effectiveness of the mAb while also delivering enough cytotoxic drugs to kill cancer cells. However, each step in the mechanism of action presents unique challenges, such as preventing unwarranted release of the cytotoxin and maximizing drug delivery. The efficiency of the internalization process and the limited availability of cell-surface antigens also make the choice of cytotoxin critical. The success of an ADC depends on several factors, including the choice of target, mAb, linker, and cytotoxin, and different classes of cytotoxic drugs that interfere with critical cell functioning to induce cell death or apoptosis. Finally, as targeted cancer cells die, neighboring tumor cells and supporting stromal tissue can also be affected by the active cytotoxic drug.
Selecting Target Antigens for ADC Development
Developing a successful ADC requires careful selection of the target antigen, which should ideally be tumor-specific or tumor-associated with high expression levels and efficient internalization. While tumor-specific antigens are preferred, most ADC targets tend to be tumor-associated, with the minimal expression on healthy tissue cells. The required level of antigen expression for effective ADC activity varies depending on the antigen in question. An optimal target antigen should also incite effective ADC internalization, and some antigens can elicit antigen-mediated antitumor activity in addition to cytotoxic activity from the conjugated drug. T-DM1 is an example of an ADC with proven anti-tumor activity resulting from the target antigen.
Types of Tumor-Associated Antigens for ADCs
ADCs can target various categories of tumor-associated antigens, including cell-surface proteins, glycoproteins, extracellular matrix proteins, and aberrant gangliosides. There is also interest in targeting antigens present in tissues that support the growth and spread of tumor cells, such as neovasculature and extracellular stromal tissue. ADCs that bind to neovasculature destroy the tumor blood supply and cause tumor cell death via nutrient deprivation and hypoxia. ADCs that target extracellular stromal tissue cause tumor cell death by reducing the concentration of growth factors produced by the stroma. Examples of anti-tumor stromal targets include fibroblast activation protein (FAP) and protein tyrosine kinase 7 (Ptk 7). ADCs that target these tissues have a wider efficacy, as all tumors depend on angiogenesis and stromal factors for their survival and growth.
Overview of Antibody-Drug Conjugates (ADCs) as Targeted Therapies for Cancer
Antibody-drug conjugates (ADCs) are targeted therapies designed to bind specifically to tumor cells and deliver a potent drug directly to the site of the tumor. The ideal ADC should have high specificity and affinity for the target antigen, low cross-reactivity with healthy tissues, a long half-life, and minimal immunogenicity. ADCs are typically made with chimerical or humanized antibodies that have a large size to limit their distribution into metabolizing organs, and their half-life is extended through binding to FcRn receptors in endothelial cells. ADCs can exert anti-tumor activity through direct modulation of the biological activity of the target antigen or via immune effectors functions. Some ADCs have direct cytotoxic effects, while others promote natural anti-tumor immune response mechanisms such as ADCC, CDC, or CDCC. Overall, ADCs have the potential to be highly effective and targeted cancer therapies.
What are the different types of linkers used in ADCs and how do they work?
Antibody-drug conjugates (ADCs) use linkers to connect the cytotoxic drug to the antibody. Linkers play a crucial role in determining the safety, specificity, potency, and activity of ADCs. Linkers are designed to be stable in the bloodstream to allow for increased circulation time and labile at the cancer site to permit the rapid release of the cytotoxic drug.
There are two broad linker designs used in ADCs: cleavable and non-cleavable linkers. Cleavable linkers exploit the differences between conditions in the bloodstream and the cytoplasmic conditions within cancer cells to trigger the release of the active drug. There are three types of cleavable linkers: hydrazone, disulfide, and peptide linkers. Hydrazones linkers undergo acid hydrolysis at low pH within lysosomes to release the cytotoxic drug. Disulfide linkers take advantage of elevated concentrations of thiol molecules (e.g., glutathione) within cancer cells to cleave the linker. Enzyme-labile or peptide linkers offer improved control of drug release by attaching the cytotoxic drug to the mAb via a dipeptide linkage, which is cleaved in the acidic environment within lysosomes by lysosomal proteases.
Non-cleavable linkers depend solely on the process of lysosomal degradation following ADC-antigen internalization. Protease enzymes within the lysosome break down the protein structure of the mAb, leaving behind a single amino acid (usually a lysine or a cysteine) still attached to the linker and cytotoxin. The resulting amino acid-linker-cytotoxin complex is released into the cytoplasm and subsequently becomes the active drug.
The choice of linker depends on various parameters, including the cleavability of the linker, the position and mechanism of linkage, and the intracellular conditions of the cancer cells. Once an optimal linker for a particular ADC is chosen, it is important to determine the ideal number of drugs to be conjugated to a mAb to achieve adequate cytotoxicity without increasing the risk of side effects, clearance, or immunogenicity.
Antibody-Drug Conjugates (ADCs) and their Ideal Characteristics
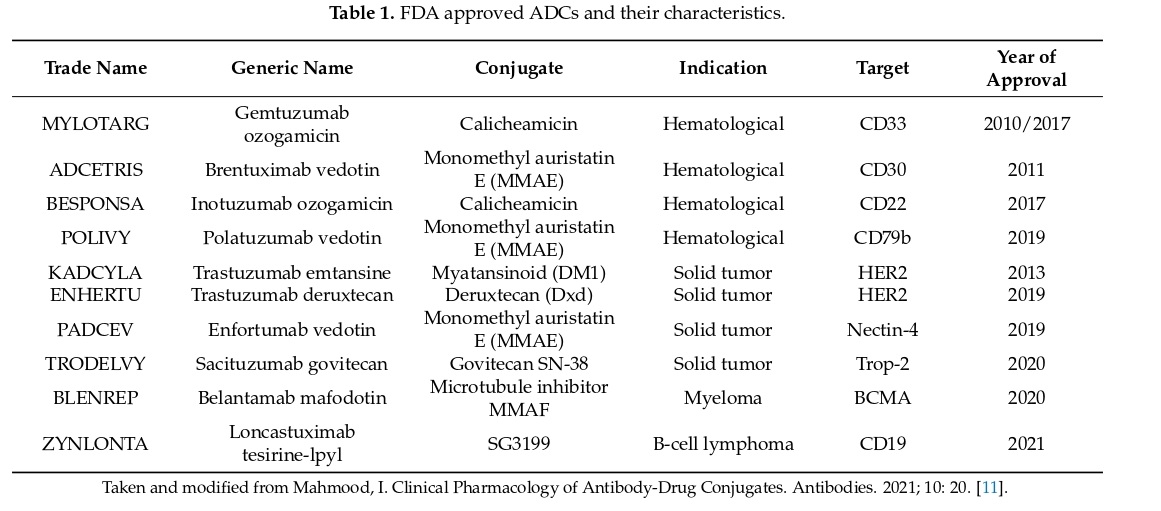
The ideal ADC targets a specific cancer antigen while sparing healthy cells uses a potent cytotoxic agent that induces cell death after being taken up by tumor cells, has a stable linker that releases the drug at the tumor site, and reduces systemic toxicity by targeting cancer cells. ADCs increase the cytotoxic potential of monoclonal antibodies, improve tumor selectivity, and are associated with lower systemic exposure compared to standard chemotherapeutic drugs. Some examples of FDA-approved ADCs are shown below.
The development of effective and safe antibody-drug conjugates (ADCs) requires an understanding of target antigen selection, internalization by cancer cells, drug potency, and linker reliability. Crucial factors in ADC development include highly potent drug conjugation techniques, achieving the optimal drug-to-antibody ratio (DAR), minimizing the impact of conjugation on antibody characteristics, and designing effective linkers.
Challenges and Strategies in ADC Development
Over the years, significant progress has been made in the development of ADC components, including linker technology, conjugation chemistry, antibody engineering, and cytotoxin identification. This has resulted in the approval of several ADCs and advanced clinical development of others. However, the cost of ADC development remains a major drawback that limits their widespread use in the clinic. Despite this challenge, efforts are being made to decrease the cost of ADC production, including the exploration of cheaper techniques to produce mAbs.
The development of antibody-drug conjugates (ADCs) encounters various challenges, including the effectiveness and safety of the treatment, resistance mechanisms that limit its efficacy, and issues related to the use of monoclonal antibodies. To enhance the therapeutic benefits of ADCs, it is important to ensure stability in the targeted antigen and signaling pathway during treatment. ADCs may face resistance mechanisms that can be genetic or non-genetic and are specific to the tumor antigen and pathway. Researchers are investigating the use of smaller antibody fragments to improve tumor penetration, and developing linker chemistry techniques to yield homogeneous ADC mixtures. However, the choice of cytotoxins for ADCs is limited by the need for potent, soluble, and linkable drugs that allow for optimal drug-to-antibody ratio (DAR). Most cytotoxins are susceptible to resistance due to the overexpression of non-specific, active transporters, and efforts are being made to overcome this limitation and increase intracellular concentrations of the cytotoxic drug.
Advances in ADCs and their Potential in Non-Oncology Indications
Although there are currently almost 300 ADCs in preclinical/clinical development, there is still much potential for this technology to be fully realized. Over the years, researchers and clinicians have gained a better understanding of the necessary components of ADCs and how altering them can impact efficacy and tolerability. This has led to the exploration of using ADCs for non-oncology indications, such as autoimmune disorders and microbial infections. Advances in technology, including new targets, payloads, conjugation techniques, antibody formats, and linkers, will enable a new generation of targeted therapeutics with an increased therapeutic window. The field is constantly evolving and adapting based on clinical and research findings, ultimately benefiting patients.
References
- Trail, P.A. (2013), “Antibody-drug conjugates as cancer therapeutics”. Antibodies 2, 113–129.
- Goldmacher, V.S. and Kovtun, Y.V. (2011),” Antibody-drug conjugates: using monoclonal antibodies for delivery of cytotoxic payloads to cancer cells”, Ther. Deliv. 2, 397–416.
- Casi, G. and Neri, D. (2012),” Antibody-drug conjugates: basic concepts, examples, and future perspectives. J. Control Release”, 161, 422–428.
- Leal, M., Sapra, P., Hurvitz, S.A., Senter, P., Wahl, A., Schutten, M., Shah, D.K., Haddish-Berhane, N. and Kabbarah, O. (2014), “Antibody-drug conjugates: an emerging modality for the treatment of cancer”, Ann. N.Y. Acad. Sci. 1321, 41–54.
- Kovtun, Y.V.; Goldmacher, V.S. “Cell killing by antibody–drug conjugates. Cancer”, Lett. 2007, 255, 232–240.
- Tsuchikama, K.,” Novel Chemical Linkers for Next-generation Antibody-drugConjugates (ADCs)”, Yakugaku Zasshi 2019, 139, 209–219.



