The Clinical Laboratory Improvement Amendments (CLIA) of 1988 was introduced to regulate physician office laboratories. The CLIA regulations aimed to ensure the quality and consistency of laboratory testing, with three levels of certification based on complexity. The Clinical Laboratory Improvement Amendments (CLIA) regulates laboratory testing and categorizes tests into three main categories based on their complexity: waived tests, moderately complex tests, and high complexity tests.
Waived tests are simple to perform and carry a low risk of incorrect results. These include some of the basic tests used at patient bedside settings and are often available over the counter to consumers. Examples include urine pregnancy tests, rapid strep tests, dipsticks for urine chemistry testing, and glucometers.
Moderately complex tests are typically found in clinical laboratory instrumentation. Facilities using these types of tests need to complete a proper instrument validation process to show proof of accurate testing, which includes precision, accuracy, verification of reportable range, and reference intervals or reference range for the patient population within the geographical area. Examples include chemistry panels, complete blood counts (CBC), some molecular (PCR) testing, urine dipsticks, urine drug screens, and automated immunoassay tests.
High-complexity tests require clinical laboratory expertise beyond normal automation to perform. Examples include lipid chromatography-mass spectrometry (LCMS), cytology, flow cytometry, gel electrophoresis, and most molecular diagnostic tests that include gene chip arrays, dot blots, viral loads, expression arrays, and CGH arrays.
Laboratories performing only waived tests required the least regulatory oversight, while those performing moderately complex tests faced more cumbersome regulatory requirements. In response to these regulations, many physicians’ offices either limited or stopped performing laboratory tests on-site. Adjustments were made to the CLIA ’88 regulations in 1993 to allow providers to perform more tests with a lower category certification, including the provider-performed microscopy (PPM) classification, which falls within the waived laboratory regulations. The SHS laboratory at UTMB performs various tests that can be done with a CLIA PPM certificate, including wet mounts, potassium hydroxide preparations, finger-stick glucose determination, urinalysis, urine pregnancy tests, testing for occult blood in stools, and rapid strep screens. Rapid strep screens were once considered “moderately complex” tests but can now be done with a CLIA-waived certificate.
The CLIA’88 Inspection Process and Quality Management in Laboratories
According to CLIA’88, laboratories are required to undergo inspections every 2 years to ensure compliance with good laboratory practices. In addition to objective measurements of quality, such as those mandated by CLIA’88, subjective indicators such as inspection deficiencies and government sanctions are also taken into account. The inspection process is seen as an opportunity for laboratories to improve their quality, and laboratories that are found to have deficiencies during inspections must correct them. While most laboratories comply with this requirement, a small percentage does not, and they may face sanctions, including revocation of their CLIA certificate.
Steps for Obtaining CLIA Certification
To obtain CLIA certification, the following steps need to be taken: determine the type of certificate required based on the complexity of testing, and complete the application. After completing the CLIA application and paying the applicable fee, which can vary based on the type of certificate and the number of tests performed. Required documentation includes laboratory director qualifications and training, quality control procedures and records, and participation in a CMS-approved proficiency testing program, provide documentation of laboratory director qualifications and training, provide quality control procedures documentation, participate in a CMS-approved proficiency testing program, and pass an on-site inspection. Certification is granted upon successful completion of these steps and compliance with regulations and quality standards must be maintained to retain certification.
To keep their CLIA certification, laboratories have to meet ongoing requirements, which include regular proficiency testing and inspections. They must also update their policies and procedures to comply with new regulations and stay current with new developments in laboratory science and healthcare regulations. By doing so, laboratories can continue to provide high-quality testing services and maintain their CLIA certification.
Key Considerations for Setting up a Laboratory: Equipment, Certification, and Staffing
Setting up a laboratory requires an initial investment to purchase equipment and supplies such as centrifuges, glucometers, and microscopes. A CLIA certificate costing $200 needs to be obtained and renewed every two years. The laboratory director may also serve as the clinic’s medical consultant, and the staff needs to be trained while policies and procedures are developed. The laboratory director is responsible for updating personnel skills and completing biannual CLIA QA paperwork. UTMB SHS laboratory staff found that on-site testing takes the same amount of time as sending specimens to an outside laboratory. Providers may spend more time performing tests if paraprofessionals usually process specimens, and the clinic nurse may have increased responsibilities for external controls on reagents.
Sources for CLIA Laboratory Policies and Procedures
The policies for CLIA laboratories can be found on the CDC website, and if an SHS (Student Health Services) is affiliated with a hospital, they can adapt their policies to meet their needs. Procedure manuals from a clinic’s reference laboratory or package inserts of commercial tests can be used as a reference for specific laboratory procedures. Hospital laboratories have stricter guidelines for documentation compared to a CLIA laboratories. Utilizing community or university resources can save time and effort.
Quality Assurance in Laboratory Testing
The staff should maintain documentation for reagents, quality control, and testing results to ensure consistent and reproducible outcomes. Documentation forms can be obtained from the hospital or SHS laboratory. The lot number, expiration date, and date opened must be recorded for reagents. Quality control involves external control testing, and results should be recorded in the patient’s chart and logbook. To ensure reproducibility, tests should be reexamined by another properly trained staff member, and evidence should be recorded. Moderately complex laboratories or higher must conduct proficiency testing.
The image below presents the analyte-specific tolerance limits for selected chemistry analytes as mandated by CLIA ’88. These tolerance limits are comprehensive measures of total error that incorporate both inaccuracy and imprecision. They are defined as either fixed limits or multiples of the PT group standard deviation.
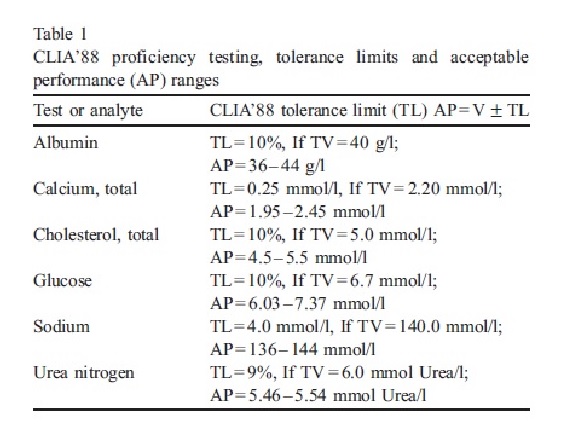
Each PT event involves five samples for an analyte, and achieving at least four out of five correct results (i.e., >80% correct performance) is considered acceptable performance. However, if there is more than one incorrect result (i.e., <80% correct), the analyte is considered failed for that PT event.
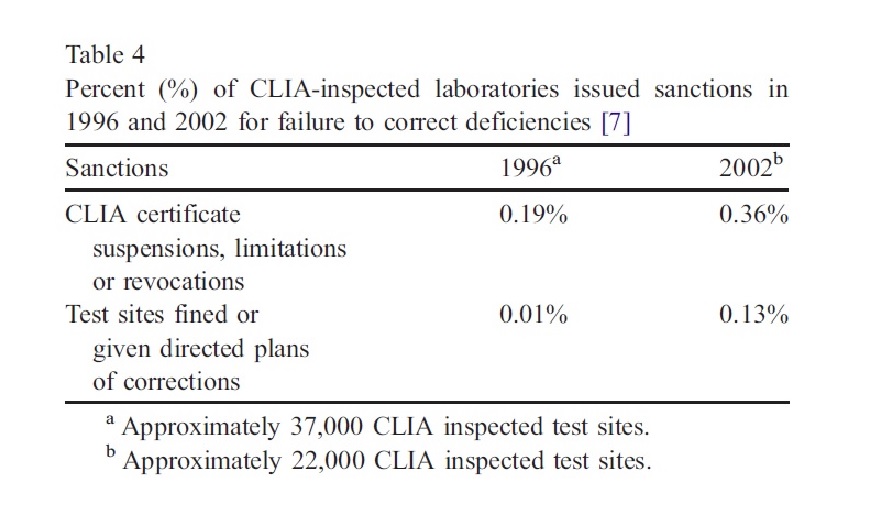
The indicators related to CLIA’88 implementation in laboratories, such as government sanctions and frequency of citations, increased from 1996 to 2002. However, data interpretation is difficult due to the low percentage of sanctioned laboratories for repeated violations. Inspectors believe most test sites should comply with CLIA’88 requirements. Additionally, the quality of test results is considered an indicator of CLIA’88 effectiveness. A report suggests that preventable medical errors cause up to 98,000 American deaths annually, indicating the need for improvement in medical care. CLIA’88 requirements could play a role in addressing this issue.
Quality Assurance Guidelines for CLIA Laboratories
Quality Assurance guidelines for CLIA laboratories include a review of the protocols and procedures manual every six months, as well as a review of the patient test log to ensure accuracy and timely reporting of results. The accuracy of final test reports is also reviewed every six months by randomly selecting five reports and comparing them with the results recorded in the patient’s chart. The state Department of health can provide samples of quality assurance programs that meet CLIA guidelines if a program is not already in place.
The laboratory director should review and approve protocols and procedures in the procedures manual every 6 months, with any changes recorded and approved. Test reports should be randomly checked against the original chart to ensure accuracy. While proficiency testing is not required for waived or PPM laboratories, reproducibility of test results should be demonstrated by having results checked by another provider at least twice every 6 months. The competency of existing and new personnel should be assessed annually or twice yearly, respectively, through direct observation and testing. The laboratory director should also review and assess any complaints or occurrence reports every 6 months.
The advancement of point-of-care (POC) laboratory testing technologies has provided an opportunity to improve clinical services and patient outcomes, but it has also created challenges for maintaining the quality of point-of-care testing and adhering to regulatory guidelines in many institutions.
Benefits of CLIA Certification
CLIA certification is a program of the US government that ensures clinical laboratories follow specific quality standards. The certification offers several benefits to laboratories, including improved patient safety and quality of care. This is achieved by enforcing standardized testing procedures and quality control measures to ensure accurate test results.
Moreover, the certification gives recognition and credibility to the laboratory and its commitment to providing accurate and reliable test results. CLIA-certified laboratories are also eligible to participate in federal healthcare programs like Medicare and Medicaid, which can increase the patient base and revenue streams.
Compliance with CLIA regulations is a legal requirement for all clinical laboratories that perform testing on human specimens, which helps them avoid penalties and legal action. Additionally, CLIA certification requires laboratories to implement standard operating procedures and quality control measures, which can improve laboratory efficiency and reduce errors.
Future Challenges for Clinical Labs for CLIA Certification
Some of the future challenges for clinical labs for CLIA certification include:
Keeping up with technological advancements: Clinical labs must keep pace with technological advancements to remain relevant and provide quality services to patients.They must have the necessary equipment and expertise to perform advanced testing procedures.
Data management and analysis: Clinical labs generate a large amount of data, which must be efficiently managed and analyzed.They must have robust data management systems to ensure the accuracy, integrity, and security of patient data.
Compliance with regulatory requirements: Clinical labs must comply with the CLIA regulations to maintain certification. They must adhere to the standards set by the Centers for Medicare & Medicaid Services (CMS) and other regulatory bodies.
Staff training and education: Clinical lab staff must be well-trained and knowledgeable about new technologies and testing procedures. They must be able to interpret results accurately and provide reliable patient care.
Quality assurance and quality control: Clinical labs must have robust quality assurance and quality control programs to ensure the accuracy and reliability of test results. They must perform regular inspections, calibrations, and validations to maintain the integrity of their testing processes.
Financial sustainability: Clinical labs must maintain financial sustainability to remain operational. They must carefully manage costs and ensure adequate revenue streams to invest in new technology and staff training.
References
- Kathleen A. Nash MSN, RNC, FNP & Ann Ross BSN, RN (1999) Setting Up a CLIA-Certified Laboratory in a Student Health Services Clinic, Journal of American College Health, 48:3, 138-140, DOI: 10.1080/07448489909595686.
- S. Department of Health. Education and Welfare, Public Health Service, Center for Disease Control, 1967: Clinical Laboratories Improvement Act of 1967. Part F of Title III of the Public Health Service Act. Sec.353. 68. Atlanta: CDC; 1967.
- Ross J. Evolution of evaluation criteria in the College of American Pathologists Surveys. Arch Pathol Lab Med 1988; 112: 334– 9.
- Laessig R, Ehrmeyer S. Use of computer modeling to predict the magnitude of interlaboratory error tolerated by proposed CDC interlaboratory proficiency testing performance criteria.
- Clin Chem 1988; 34: 1849– 53. Committee on Quality of Health Care in America. Institute of Medicine. In: Kohn L, Corrigan J, Donaldson M, editors. To Err is Human: Building a Safer Health system. Washington, DC: National Academy Press; 2000, p. 1 –287.



