Introduction
Immunoassays are laboratory tests that use antibodies to detect and measure various substances in biological samples such as blood, urine, or saliva. Its commonly used in clinical diagnostics, drug discovery, and basic research to detect hormones, drugs, proteins, and infectious agents, among others. Some of the most commonly used immunoassays include enzyme-linked immunosorbent assays (ELISAs), radioimmunoassays (RIAs), fluorescence immunoassays (FIAs), and chemiluminescence immunoassays (CLIAs).
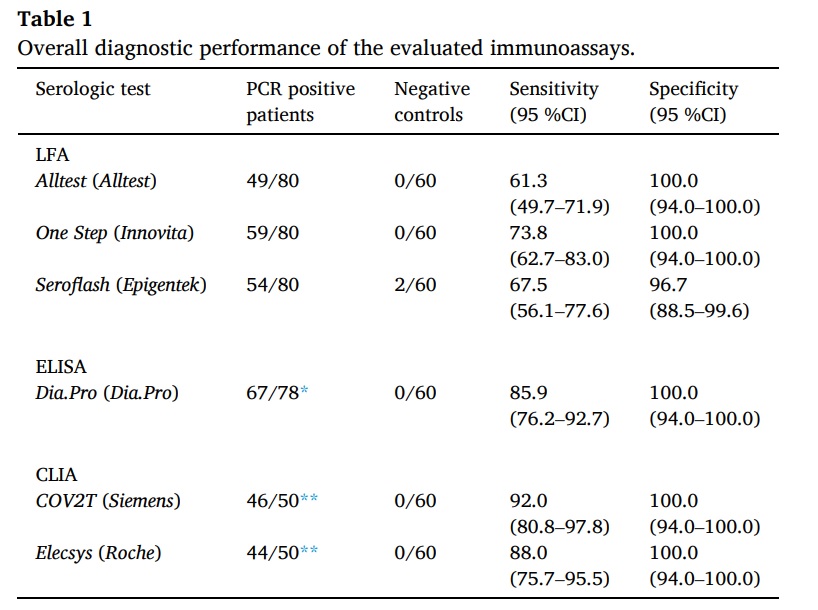
A study showed six immunoassays as reliable in detecting antibodies against SARS-CoV-2. Among these assays, LFAs that use S and N proteins as antigens have achieved the highest sensitivity. The One Step LFA, Dia. Pro ELISA, Elecsys, and COV2T CLIAs have all shown 100% specificity. Additionally, these four assays have shown a sensitivity of over 97% from 14 days after the onset of symptoms. They have also exhibited excellent agreement with kappa scores (k) greater than 0.9.
Significance of Immunoassays in Diagnostics and Research
Immunoassay techniques have found widespread use in various crucial fields of pharmaceutical analysis, such as disease diagnosis, therapeutic drug monitoring, clinical pharmacokinetic studies, and bioequivalence investigations in drug discovery and pharmaceutical industries.
Comparison of ELISA with other immunoassays
-
Radioimmunoassay (RIA)
Radioimmunoassay (RIA) is a technique that uses radiolabeled antigens to measure the concentration of substances in body fluids. A radioisotope is attached to an antigen and bound to its complementary antibody. A sample with the target antigen competes with the radioactive antigen and replaces it. The radioactive signal of the sample is measured, and its intensity is directly proportional to the target antigen concentration. RIA requires a sample with antigen, complementary antibody, and radiolabeled antigen.
Immunoassays rely on detectable labels, such as radioisotopes or enzymes, that are linked to one of the immunoanalytical reagents, such as the analyte or antibody. The incorporation of these labels in immunoassays leads to the development of highly sensitive detection systems with very low limits of detection.
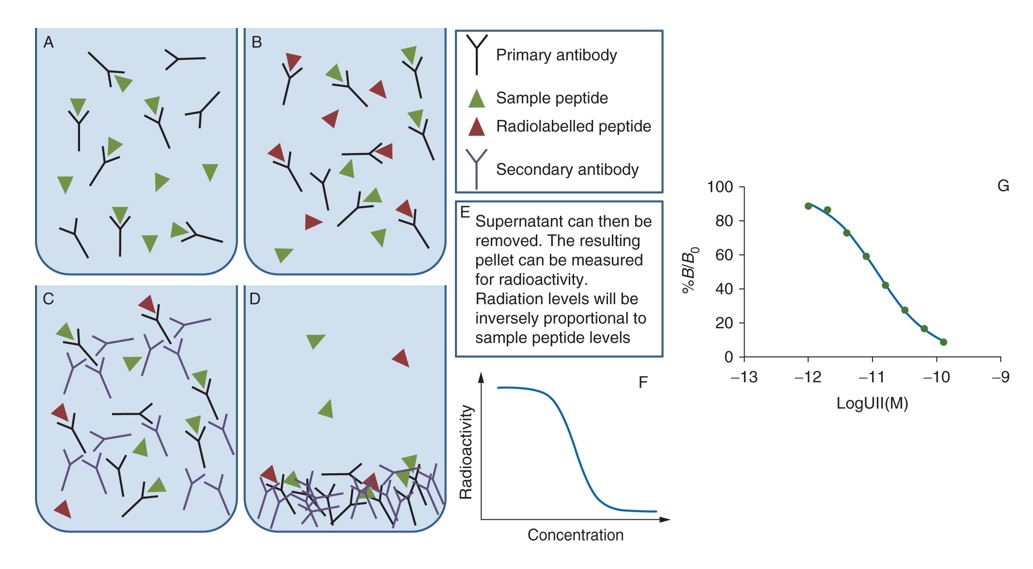
Fig 1 includes (A) Sample peptide incubated with primary antibody. (B) A radiolabelled peptide is then added. It competes with sample peptide and displaces it. (C) A secondary antibody binds to the primary antibody and causes it to precipitate out of the solution. (D) Centrifugation causes the antibody–antigen complex to form a pellet. (F) Example of a typical standard curve. (G) Actual standard curve for urotensin-II (UII) where the amount of radioactive iodine bound is expressed as B/B0 which is the ratio of binding at each standard concentration, B to that bound in the absence of displacer, B0. Analyte samples in biological specimens should lie on the straight part of the curve.
Advantages:
- High sensitivity and specificity for accurate detection and quantification of low levels of target molecules in complex biological samples
- Adaptable to a wide range of analytes including hormones, drugs, and infectious agents.
Disadvantages:
- Use of radioactive substances requiring special handling and disposal procedures.
- Specialized equipment and technical expertise lead to higher costs and time consumption.
- Replaced by other immunoassay techniques that are safer and easier to use.
Comparison with ELISA
- RIA uses a radioactive label, while ELISA uses an enzyme label.
- RIA is more sensitive but requires special handling and is more expensive, while ELISA is safer, faster, and more cost-effective.
- RIA is better for detecting low levels of antigens or antibodies in complex biological fluids, while ELISA is better for testing large numbers of samples quickly.
- The choice of the assay depends on the specific needs of the experiment, such as sensitivity, cost, and safety.
Fluoroimmunoassay (FIA)
FIA is a technique that uses fluorescent compounds to detect and measure different compounds. It is highly sensitive and fast compared to other methods, making it a popular choice in the field of in-vitro diagnostics.
In FIA, fluorescent probes are used to label antibodies, which illuminate under UV light to detect specific antigen-antibody binding. The antigen-antibody complexes are then isolated and the fluorescent intensity is measured.
A study was conducted to lower the detection limit for casein in foods using three competitive assays: direct and indirect TR-FIA, and ELISA. The sensitivity of both TR-FIAs was considerably less than ELISA. However, all three assays allowed quantification of casein in foodstuffs in the order of 1-1.5 mg kg−1, providing a basis for more rigorous validation and collaborative testing. The TR-FIA approach did not provide any improvement over the ELISA.
Advantages:
- Fast and highly sensitive for detecting and quantifying compounds
- Uses specific and highly sensitive fluorescent compounds as detection reagents
- Can be used in different settings, including in vitro diagnostics
Disadvantages:
- Requires specialized equipment for fluorescent detection
- Has a limited dynamic range of detection compared to other methods
- There may be possible interference from the sample matrix and autofluorescence.
Comparison with ELISA
The use of TRFIA (Time-Resolved Fluoro immunoassay) with monoclonal antibodies labeled with Eu3+ chelates for detecting trace amounts of sulfonamides in environmental waters. This method has low detection limits, and high selectivity, and is tolerant to variations in pH, salinity, and the presence of other substances. TRFIA is cost-effective, has a high sample throughput, and can be used without pretreatment. The study shows that TRFIA is a promising method for routine screening of sulfonamides in environmental waters, offering advantages over existing methods.
Chemiluminescent immunoassay (CLIA)
CLIA uses a chemical reaction to create light and detect the presence of a target molecule. A specific antigen or antibody is immobilized onto a solid support, and a chemiluminescent substrate is added along with a secondary antibody that binds to the target molecule.
Magnetic-bead-based CLIA is a variation that uses magnetic beads coated with a specific antigen or antibody, which are functionalized with magnetic nanoparticles to efficiently separate the target molecule from the sample.
Magneto-actuated chemiluminescence assays for Zika virus detection use magnetic beads functionalized with specific antibodies against the virus, which are subjected to a magnetic field to increase the assay’s sensitivity.
Advantages of chemiluminescent immunoassay (CLIA) include:
- High sensitivity and specificity: CLIA can detect target molecules at very low concentrations and has a low rate of false positives and false negatives.
- Wide dynamic range: CLIA can measure a broad range of concentrations of the target molecule, from very low to very high.
- Flexibility: CLIA can be used to detect a variety of different target molecules, including proteins, nucleic acids, and small molecules.
- Automation: CLIA can be easily automated, making it a convenient and efficient technique for high-throughput analysis.
- Stability: The chemiluminescent signal produced by CLIA is stable and can be measured over a longer period compared to other immunoassay techniques.
Disadvantages of chemiluminescent immunoassay (CLIA) include:
- Cost: CLIA reagents and instruments can be expensive, which may limit their use in certain applications.
- Complexity: CLIA requires specialized equipment and expertise, which can make it difficult to implement in some laboratories.
Comparison with ELISA
The two immunoassays have demonstrated consistency in their analysis, but there are variations in the quantitative results that may be attributed to the use of dissimilar standard calibrators. Although the CLIA test displays greater fluctuations in the values obtained, it is beneficial because it is an automated process and has a short waiting period. Consequently, both immunoassays can be used interchangeably for the precise determination of the Anti-HBs level in a sample.
Conclusion
In summary, ELISA is a cost-effective and widely used method but may have limitations in sensitivity and specificity. RIA is highly sensitive but more expensive and poses safety concerns due to the use of radioactive isotopes. CLIA is highly sensitive, specific, and automated with a fast turnaround time, making it a popular choice in clinical laboratories.
Reference
1. ” Immunoassay Methods and their Applications in Pharmaceutical Analysis: Basic Methodology and Recent Advances”, Ibrahim A. Darwish, Int J Biomed Sci.
2. Pieniaszek HJ, Davidson AF, Walton HL, Pinto DJ, et al. J. Pharm. Biomed. Anal. 2003;30:1441–1449. [PubMed]
3. Suzuki Y, Arakawa H, Maeda M. Anal. Sci. 2003;19:111–115. [PubMed]
4. “Comparative evaluation of six immunoassays for the detection of antibodies against SARS-CoV-2”, Author links open overlay panel Felipe Pérez-García, Ramón Pérez-Tanoira, Journal of Virological Methods
5. “Radioimmunoassay, enzyme and non-enzyme-based immunoassays “, R. D. Grange, J. P. Thompson, D. G. Lambert BJA: British Journal of Anaesthesia
6. “A comparison of time-resolved fluoro immunoassay and ELISA in the detection of casein in foodstuffs”, G. B. Sletten, K. E. Lovberg et.al
7.“Time-Resolved Fluoroimmunoassay as an Advantageous Approach for Highly Efficient Determination of Sulfonamides in Environmental Waters”, Zhen Zhang et.al,
8. “Comparing Assay Performance of ELISA and Chemiluminescence Immunoassay in Detecting Antibodies to Hepatitis B Surface Antigen”, Mridula Madiyal,1 Siddharth Sagar,2 Shashidhar Vishwanath,3 Barnini Banerjee,4 Vandana Kalwaje Eshwara,5 and Kiran Chawla



