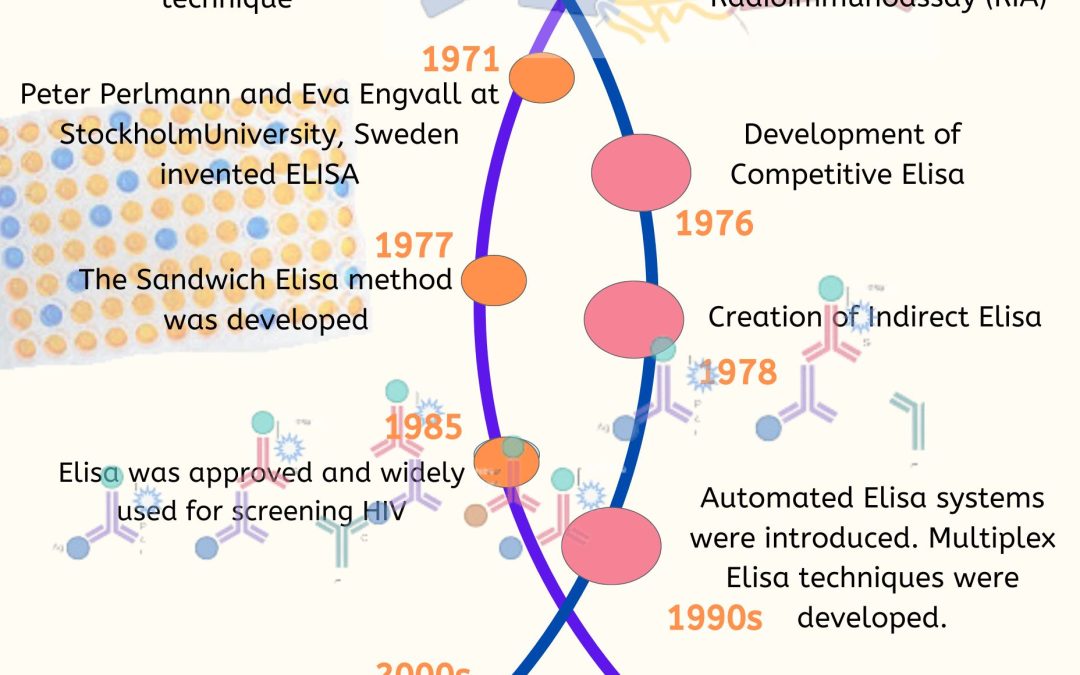
Key Milestones
1941 – Introduction of Immunofluorescence
- In 1941, Albert H. Coons and his team were the first to label antibodies with a fluorescent dye, pioneering a technique that allowed for the visualization and identification of antigens in tissue sections.
- This method, known today as immunofluorescence, marked a significant advance in immunoassay technology. By attaching fluorescent molecules to antibodies, researchers could use ultraviolet light to detect the presence and distribution of specific antigens within biological tissues, revolutionizing diagnostic and research applications in immunology.
1960 – Development of Radioimmunoassay
- Rosalyn Sussman Yalow and Solomon Berson introduced radioimmunoassay (RIA) in 1960. This technique utilized radioactive isotopes to label antibodies or antigens, enabling the detection of minute quantities of biological substances with high sensitivity.
- Although RIA represented a breakthrough, its reliance on radioactive materials posed significant health risks and environmental concerns. The need for a safer and equally effective alternative spurred further innovations in immunoassay technology.

1971 – Invention of ELISA
- In 1971, Eva Engvall and Peter Perlmann independently developed a method that would revolutionize diagnostic medicine: the Enzyme-Linked Immunosorbent Assay (ELISA).
- This technique replaced radioactive labels with enzymes, which, upon binding to a substrate, produced a detectable signal, typically a color change.
- ELISA provided a safer, simpler, and more versatile alternative to RIA, making it possible to detect and quantify hormones, viruses, and other antigens in various samples with high specificity and sensitivity.
1976 – Development of Competitive ELISA
- The competitive ELISA method was introduced in 1976. In this assay format, a known amount of an enzyme-conjugated substrate competes with the antigen of interest for binding to a specific antibody.
- The decrease in signal indicates the presence of the antigen in the sample. This method was applied to detect human chorionic gonadotropin (hCG), a hormone important in pregnancy testing. The competitive ELISA allowed for measuring small molecules and hormones with high precision.
1977 – Introduction of Sandwich ELISA
- The sandwich ELISA method was developed in 1977. In this format, the capture antibody is first coated onto the plate surface.
- The antigen from the sample binds to this antibody, and a second detection antibody, which is enzyme-conjugated, is added to form a “sandwich” with the antigen in the middle.
- This method was tested on various substrates to demonstrate its proof of concept. Sandwich ELISA is highly specific and sensitive, making it ideal for detecting and quantifying antigens in complex samples.
1978 – Creation of Indirect ELISA
- In 1978, the indirect ELISA method was created. This assay involves coating the plate with the antigen, and then adding a primary antibody specific to the antigen.
- A secondary antibody, which is enzyme-linked and specific to the primary antibody, is then added for detection. This method amplifies the signal and increases the assay’s sensitivity. Indirect ELISA was used to detect human serum albumin, showcasing its effectiveness in antibody detection and characterization.

1985 – ELISA Test for HIV Screening
- The ELISA test became the first widely used screening test for HIV in 1985. Approved for use on March 2, 1985, this test enabled the detection of antibodies against the human immunodeficiency virus (HIV) in blood samples.
- The introduction of ELISA for HIV screening marked a critical milestone in public health, providing a reliable and accessible method for diagnosing and controlling the spread of HIV/AIDS.
- ELISA saw widespread adoption in clinical laboratories for diagnosing various infectious diseases, autoimmune disorders, and allergies.
- The Sandwich and Indirect ELISA formats gained popularity due to their specificity and adaptability in detecting a range of antigens and antibodies.
1990s – Automation, Multiplexing, High-Throughput
- Automated ELISA systems were introduced, featuring robot-assisted liquid handling and microplate readers. These innovations increased efficiency and minimized human error.
- Multiplex ELISA techniques were developed, enabling the simultaneous detection of multiple analytes within a single sample. In the 1990s, technological advancements enabled the development of high-throughput multiplex immunoassays, laying the foundation for their widespread adoption in biomedical research and clinical diagnostics in subsequent decades.
- ELISA became integral to high-throughput screening processes in drug discovery, allowing for the evaluation of thousands of compounds for potential interactions with specific target proteins.
2000s – CLIA, ELFA, Microfluidics
- The development of Chemiluminescence Immunoassays (CLIA) and Enzyme-Linked Fluorescent Assays (ELFA) provided higher sensitivity and a broader dynamic range compared to traditional ELISA.
- Incorporation of microfluidics technology into ELISA, transforming the traditional 96-well format into portable, point-of-care testing devices.
2010s – Digital ELISA, Multiplexing, Integration
-
- The advent of digital ELISA enabled single-molecule detection, significantly improving the assay’s sensitivity for detecting low-abundance biomarkers.
- Further improvements in multiplexing technologies allowed for the comprehensive profiling of multiple biomarkers in both research and clinical diagnostics.
- ELISA began to be combined with mass spectrometry and next-generation sequencing, facilitating more detailed and high-throughput analyses.
- Serological diagnostic techniques like ELISA (Enzyme-Linked Immunosorbent Assay), developed in the 1970s, employ specific antibodies to detect pathogens in plant tissues, offering high throughput and low cost. Innovations like ELISA-DASI (Double Antibody Sandwich Indirect ELISA) and IC-PCR (Immunocapture Polymerase Chain Reaction) have enhanced sensitivity by combining serology with molecular assays, particularly for detecting viruses and viroids.
- As ELISA technology has evolved over the decades, researchers now have access to a wide range of complementary tools that enhance assay performance and versatility. ELISA kits provide ready-to-use solutions for detecting and quantifying antigens or antibodies, while recombinant proteins serve as precise targets for validating assay specificity. Scientists may also leverage monoclonal antibodies for highly specific antigen detection, use polyclonal antibodies to capture broader analyte profiles, or incorporate secondary antibodies to amplify signals in various assay formats. Together, these resources support the continued innovation and practical applications of ELISA in diagnostics, biomedical research, and drug discovery.
2020s: Point-of-Care, COVID-19, Automation, Safety
- Point-of-care ELISA technologies have become crucial during the COVID-19 pandemic, offering rapid and on-site detection of SARS-CoV-2 (severe acute respiratory syndrome coronavirus-2) antibodies and antigens, facilitating timely diagnosis and management in diverse healthcare settings globally.
- ELISA was extensively used to detect antibodies against SARS-CoV-2, aiding in epidemiological studies and evaluating vaccine efficacy during the COVID-19 pandemic.
- Enhanced automation and the integration of artificial intelligence improved data analysis and interpretation, increasing the accuracy and throughput of ELISA assays.
- The current evolution in tuberculosis detection methods for cattle involves automated approaches using ELISA, integrating advanced machine learning techniques like neural networks and digital signal processing. These advancements aim to enhance speed, accuracy, and efficiency in veterinary diagnostics, paving the way for more precise management and control of tuberculosis in livestock.
- ELISA saw broader application in monitoring pollutants and ensuring food safety by detecting contaminants and allergens.
These milestones highlight the evolution of immunoassays techniques and the significant impact of ELISA on medical diagnostics and research. Each advancement built upon previous innovations, leading to the development of highly sensitive, specific, and versatile assays that are now fundamental tools in biomedical science.
Explore Our Full Range of ELISA Kits
Whether you’re testing human, animal, or plant samples, MyBioSource offers over 1 million ELISA kits covering thousands of analytes across every major species.
References
- Ahsan, H. Monoplex and multiplex immunoassays: approval, advancements, and alternatives. Comp Clin Pathol 31, 333–345 (2022).
- Ghodake, Gajanan Sampatrao, et al. “Biological characteristics and biomarkers of novel SARS-CoV-2 facilitated rapid development and implementation of diagnostic tools and surveillance measures.” Biosensors and Bioelectronics 177 (2021): 112969.
- Hanene Sahli, Aymen Mouelhi, Mohamed Fethi Diouani, Lotfi Tlig, Amira Refai, Ramzi Boubaker Landoulsi, Mounir Sayadi, Makram Essafi, An advanced intelligent ELISA test for bovine tuberculosis diagnosis, Biomedical Signal Processing and Control, Volume 46, 2018, Pages 59-66, ISSN 1746-8094.
- Trippa, D., Scalenghe, R., Basso, M. F., Panno, S., Davino, S., Morone, C., … & Martinelli, F. (2024). Next‐generation methods for early disease detection in crops. Pest management science, 80(2), 245-261.
- Yorde, Donald, et al. “Competitive Enzyme-Linked Immunoassay with Use of Soluble Enzyme/Antibody Immune Complexes for Labeling. I. Measurement of Human Choriogonadotropin.” Clin. Chem. 1976;22/8,1372–1377 Yorde, Donald et al. “Competitive Enzyme-Linked Immunoassay with Use of Soluble Enzyme/Antibody Immune Complexes for Labeling. I. Measurement of Human Choriogonadotropin.” Clin. Chem. 1976;22/8,1372–1377
- Lindström, P. et al. “IgG autoantibody to human serum albumin studied by the ELISA-technique.” Scand J Immunol. 1978;7(5):419-25.Lindström, P. et al. “IgG autoantibody to human serum albumin studied by the ELISA-technique.” Scand J Immunol. 1978;7(5):419-25.
- Czerkinsky, C et al. “A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells.” J Immunol Methods. 1983;65 (1–2): 109–121.Czerkinsky, C et al. “A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells.” J Immunol Methods. 1983;65 (1–2): 109–121.
- Perlmann, Peter, et al. “Enzyme-linked immunosorbent assay (ELISA) quantitative assay of immunoglobulin G.” Immunochemistry. 1971;8 (9): 871–4.Perlmann, Peter, et al. “Enzyme-linked immunosorbent assay (ELISA) quantitative assay of immunoglobulin G.” Immunochemistry. 1971;8 (9): 871–4.