Monoclonal antibodies (mAbs) have become a significant therapeutic option in many diseases, and the field is becoming highly competitive with over 200 new candidates entering clinical trials. To stand out in this market, the new clinical candidate must be differentiated from its competitors or other approved antibodies. One way to achieve this is to optimize the epitomes to which the antibody binds, the affinity to the target, the pharmacokinetics, the effectors’ function of the constant region (Fc region), and the safety profile of the antibody. The variable region of the antibody can be engineered to improve these properties and generate superior antibody therapeutics. The primary role of the antibody variable region is to bind to the antigen, but it can also affect various properties important for developing antibody therapeutics.
The effector’s function of the antibody is important for both efficacy and safety and can be optimized by selecting the right isotype and various Fc engineering technologies. The immunogenicity of the antibody can be reduced by various humanization and deimmunization strategies. Efficient preclinical development of therapeutic antibodies can be facilitated by cross-reactivity of the antibody to rodent or nonhuman primate antigens, and CMC development can be hampered by the pharmaceutical properties of the antibody molecule. Various drug-related properties of mAbs can be improved by antibody engineering and optimization technologies, which can be classified into two categories: variable region engineering and Fc engineering.
Why monoclonal antibodies are to be engineered?
Monoclonal antibodies need to be engineered to increase their specificity, potency, and therapeutic efficacy. This is because naturally occurring antibodies may not be suitable for clinical use due to their low affinity for the target antigen or the presence of undesirable effectors functions. Engineering techniques such as humanization, affinity maturation, and Fc region modification can improve the pharmacokinetic and pharmacodynamic properties of monoclonal antibodies. These modifications can also reduce immunogenicity and increase safety, making them more effective for use as therapeutic agents.
Engineering Strategies for Improving Stability, Solubility, Viscosity, Chemical Stability, and Heterogeneity
Various engineering technologies have been developed to improve the stability of the variable region sequence of an antibody, which greatly affects its pharmaceutical properties. Poor stability can increase manufacturing costs due to issues such as decreased solubility and chemical stability. To improve stability, engineering methods such as rational mutagenesis and library approaches have been used to optimize key residues. For scFv, selecting the VH3 germ line framework and ensuring CDR compatibility with framework residues has shown promise. While stability engineering of the Fab domain in IgG antibodies has not been extensively researched, it may be necessary for poorly performing antibodies. Antibodies with a low melting temperature are more likely to aggregate and express poorly.
Subcutaneous delivery of antibody therapeutics is preferred for chronic diseases but requires a formulation with high antibody concentration (>100 mg/mL) due to the limited volume for a single injection. However, achieving this high concentration is difficult due to issues with stability, solubility, and viscosity. To improve solubility, introducing an N-linked carbohydrate in the variable region is the most effective strategy but may increase heterogeneity derived from the additional carbohydrate, requiring more control of the heterogeneity in the manufacturing process. To improve viscosity, no variable region engineering has been reported to date. To improve chemical stability, different degradation pathways such as deamidation, isomerization, succinimide formation, methionine and tryptophan oxidation, and cysteinylation of unpaired cysteine in the CDR region often lead to a reduction in the potency of antibody therapeutics and could be issues needing to be controlled in CMC development. Finally, to improve heterogeneity, heterogeneities deriving from post-translational modification, such as glycosylation, N-terminal pyroglutamine cyclization, and C-terminal clipping need to be controlled to maintain the product quality.
Monoclonal Antibodies: Structure, Classification, and Effectors Functions
Antibodies are large proteins composed of two identical light chains and two identical heavy chains, which are categorized into five classes based on their heavy chain sequences. All clinically used therapeutic monoclonal antibodies (mAbs) are IgGs due to their relatively easy production and prolonged circulating half-life. The antigen-binding fragment (Fab) of mAbs is composed of heavy and light variable chains, and the paratope, which is unique to each mAb, defines its target specificity. The crystallizable or constant fragment (Fc) region determines the effector’s function of mAbs by binding to Fc gamma receptors (FcγR) on immune cells, initiating complement-dependent cytotoxicity (CDC) and antibody-dependent cellular cytotoxicity (ADCC). Isotypes IgG1 and IgG3 are the most potent activators of the classical complement pathway and are effective at promoting ADCC. In contrast, IgG2 and IgG4 subtypes have reduced effectors function, which is preferable for immunological indications where ADCC or CDC is not desirable.
Approaches for Engineering Monoclonal Antibodies
Fc Engineering
FC engineering refers to modifying the Fc region of monoclonal antibodies to enhance their therapeutic properties in biopharmaceutical manufacturing. This region is crucial for determining the antibody’s function and interacts with immune cells and other molecules in the body. Various modifications can be made to the Fc region, such as changing the glycosylation pattern, adding chemical groups, or introducing mutations. These modifications can impact the antibody’s ability to bind to specific receptors on immune cells, modulate the immune response, and increase stability and half-life in the body. A common method of FC engineering involves creating Fc-fusion proteins, which can improve pharmacokinetics, and efficacy, and enable new modes of administration. FC engineering is an active area of research and development in the biopharmaceutical industry, and it plays a crucial role in improving the therapeutic properties of mAbs and other biopharmaceuticals.
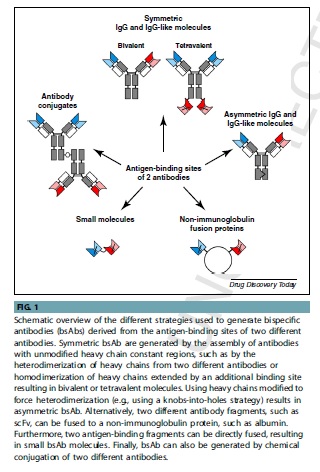
Bispecific Antibodies
Bispecific antibodies (bsAbs) have revolutionized therapeutic and diagnostic applications. Initially produced through chemical conjugation or fusion of two hybridomas, over 50 recombinant bsAb formats have been developed through genetic engineering. BsAbs can have or lack an Fc region, with the former showing better stability and effectors functions. BsIgG molecules can be generated by the fusion of different heavy and light chains but this can lead to non-functional molecules. Tetravalent molecules with two binding sites for each antigen can be obtained by fusing a second binding moiety. Asymmetric antibodies generated through knobs-into-holes technology can suffer from the “light-chain problem.” CrossMab technology can address this problem by exchanging the CH1 domain of one heavy chain with the CL domain of the corresponding light chain. Asymmetric heavy chains can also be used to generate trivalent, bispecific, or even tri-specific antibodies by fusing a further antigen-binding moiety into one of the heavy chains.
Antibody-Drug Conjugates
Chemotherapy is effective in treating tumors but has limitations, such as lack of specificity and high toxicity. Targeted treatments like tumor antigen-specific antibodies have limitations, including limited tissue penetrance and resistance. Antibody-drug conjugates (ADCs) combine monoclonal antibodies’ selectivity with cytotoxic potential to deliver drugs directly to cancer cells. ADCs work by binding to the target antigen and releasing the drug payload. The majority of ADCs have on-target on-tumor effects, but some can also cause off-target on-tumor cell killing, known as the bystander effect. Non-internalizing ADCs have a perceived risk of killing immune cells, while internalizing ADCs are at the forefront of developing targeted anti-cancer therapeutics.
Antibody Fragments
There is growing interest in expressing various forms of monoclonal antibodies due to recent successful clinical results. Large doses are often required for therapeutic purposes, leading to the development of efficient and cost-effective production systems. Truncated forms of antibodies, including Fabs, Fab’2, and single-chain Fv forms, have been used for clinical applications and can be generated from mammalian-produced full-length antibodies. Escherichia coli is the preferred production system for antibody fragments used in therapeutic applications due to its rapid cell line production and high production levels. Several approaches have been taken to improve production levels, including optimizing expression, host-cell engineering, and co-expressing chaperones. Techniques such as site-specific attachment of PEG moieties and albumin-binding peptides have been developed to increase the circulating half-life of antibody fragments. Recent reports show that full-length antibodies can be produced in E. coli, providing a potential new option for therapeutic indications that do not require effectors functions.
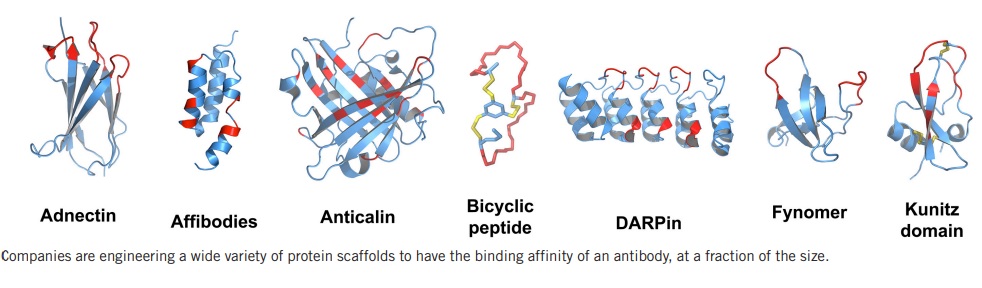
Antibody-like Scaffolds
Protein scaffold drugs are a new class of therapeutic molecules that use tiny protein structures to mimic the high binding specificity of antibodies while overcoming some of their limitations. Protein scaffolds are much smaller than antibodies, making them more effective in penetrating solid tumors or creating inhaled drugs. They are also more stable at high temperatures and can be produced using bacteria, yeast, or chemical synthesis. Moreover, scaffolds are different enough from antibodies that they can be patented. However, these intellectual property and cost advantages are beginning to erode as patents for antibody drugs expire and new manufacturing techniques drive down the cost of biologics drug production. Protein scaffolds’ small size also means they have a short half-life, which could limit their efficacy. Only one antibody-like scaffold drug has made it to market, and the rest of the pipeline is moving slowly. Nonetheless, there is still considerable interest in the development of these drugs due to their potential advantages over traditional antibodies.
Clinical Applications of Engineered Monoclonal Antibodies
Cancer Immunotherapy
Antibody engineering has created large libraries of antibodies for cancer therapy, which can be screened in vitro to select antibodies with specificities. Phage display is the most popular selection method, where variable genes are fused to bacteriophage coat proteins, and antibodies are selected against an immobilized target. Recombinant antibodies can be expressed in different forms, such as dimers and trimers, with better tumor penetration than intact antibodies. Bispecific antibodies can bind two antigens and redirect effectors cells to target cells, with a bias towards producing heterodimers.
Autoimmune Diseases
Current treatments for autoimmune disorders such as RA (Rheumatoid arthritis) focus on relieving pain, reducing inflammation, and suppressing the immune response. However, commonly used drugs such as NSAIDs and corticosteroids have significant side effects such as gastrointestinal toxicity and weight gain. Disease-modifying anti-rheumatic drugs (DMARDs) are also effective but take months to show positive effects and cause non-specific side effects. To address these issues, there is a shift toward developing drugs that target the causes of the disease rather than symptoms. Biological response modifiers (BRMs) such as Enbrel, Remicade, and HUMIRA directly target cytokines like TNF-α, which are involved in the development and progression of RA. These BRMs have demonstrated efficacy in clinical trials and are particularly suited to the role due to their ability to bind specifically and with high affinity to a wide range of biological molecules, as well as having a longer half-life than small-molecule treatments.
Infectious Diseases
A new approach for treating infectious diseases using antibody cocktails has been developed by scientists. This method involves combining several antibodies that target multiple antigens and epitopes, providing broad protection against a variety of strains using multiple mechanisms. Antibodies with high affinity, neutralizing properties, and targeting different conserved epitopes are selected to reduce the risk of escape variants. Even though cocktails are currently more expensive than monoclonal therapies, new manufacturing strategies are being developed. Antibody cocktails are promising for highly mutable pathogens like HIV, where mixtures of antibodies targeting distinct epitopes protect against a broader range of circulating strains. Cocktails have also been explored to target multiple bacterial virulence factors, such as anti-botulism toxin and pertussis toxin, which showed efficacy in treating established diseases in baboons. These cocktails include neutralizing antibodies that target different subunits of the toxin. Similarly, two antibodies targeting the staphylococcal enterotoxin B superantigen act via complementary mechanisms.
Future directions
Monoclonal antibody engineering is advancing, with two areas of research standing out: the creation of bispecific or multi-specific antibodies that can target multiple antigens and immune cells, and the development of antibody-drug conjugates that combine antibodies with the cytotoxicity of drugs. These advancements could be particularly useful for treating complex diseases such as cancer. Additionally, research is underway to improve the safety of monoclonal antibodies by reducing the risk of adverse effects and immunogenicity. The ongoing improvements in monoclonal antibody engineering show potential for enhancing the efficacy and safety of these agents.
References
- Franey H, Brych SR, et.al; “Increased aggregation propensity of IgG2 subclass over IgG1: role of conformational changes and covalent character in isolated aggregates”, Protein Sci 2010; 19:1601-15.
- Geng X, Kong X, Hu H, et al. Research and development of therapeutic mAbs: an analysis based on pipeline projects. Hum Vaccin Immunother. 2015; 11:2769‐2776.
- Ewert S, Huber T, Honegger A, Plückthun alanine, “Biophysical properties of human antibody variable domains”, Mol Biol 2003; 325:531-53.
- Ewert S, Honegger A, Plückthun A.,” Stability improvement of antibodies for extracellular and intracellular applications: CDR grafting to stable frameworks and structure-based framework engineering”, Methods 2004; 34:184-99.
- Röthlisberger D, Honegger A, Plückthun A. Domain interactions in the Fab fragment: a comparative evaluation of the single-chain Fv and Fab format engineered with variable domains of different stability. J Mol Biol 2005; 347:773-89.
- Peters C, Brown S. “Antibody-drug conjugates as novel anti-cancer chemotherapeutics”, Biosci Rep. 2015; 35:e00225. Doi: 10.1042/BSR20150089. PMID: 26182432.