Monoclonal antibodies (mAbs) are laboratory-produced molecules that can substitute antibodies and bind to antigens found on cancer cells. The development of mAbs has been in progress for over a century, starting with the discovery by Dr. Edward Jenner in the 18th century that injecting fluid from a smallpox pustule could provide immunity. In the 1930s and 1940s, scientists contributed to the concept of mAbs, and the first laboratory-created mAbs were published in 1970. The first monoclonal antibody for human use was generated in 1975, and the first fully licensed mAb was approved in 1986. The initial mAbs were created using mouse proteins and were not well-tolerated in humans, leading to the development of fully humanized antibodies. The first mAb approved by the FDA was muromonab-CD3, which was used as an anti-rejection medication. The first mAb found successful in treating solid tumors was trastuzumab, which was initiated by the humanized human epidermal growth factor receptor 2 (HER2). The development of trastuzumab also led to the beginning of drug discovery and treatment in cancer targeting a patient’s unique biomarkers.
Importance of Immune Response Modulation
The immune system is vital for fighting infections and diseases, but it can also have harmful effects when it is too active or too weak. Modulating the immune response is a crucial strategy for preventing or treating diseases. This involves using drugs or therapies to regulate the immune system’s activity to enhance or dampen it, depending on the condition. Immune response modulation is essential for developing vaccines and cancer immunotherapy, which work by stimulating the immune system to develop immunity against specific infections and attack cancer cells. Overall, immune response modulation is essential for preventing and treating diseases, including autoimmune disorders, infections, cancer, and transplant rejection.
Neutralization
MAbs play a crucial role in neutralizing pathogens and toxins by preventing them from infecting or damaging cells in the body. This is achieved by mAbs binding to specific receptors on the pathogen or toxin, hindering its ability to enter cells. For viruses like SARS-CoV-2, neutralizing mAbs target the spike protein on the virus’s surface. Neutralizing mAbs are also used to treat diseases caused by toxins, like botulism and tetanus. The function of neutralizing mAbs is vital in the development of treatments and prevention strategies for infectious and toxin-mediated diseases. In conclusion, neutralizing mAbs has far-reaching implications for public health.
The COVID-19 pandemic has led to the development and repurposing of various preventive treatments to fight against the virus. One promising class of antiviral interventions is mAbs, which can attach to and inactivate the virus in infected individuals. These neutralizing mAbs are synthetic proteins that can be produced from the B cells of patients who have recovered from COVID-19 or humanized mice.

Over 125 years ago, a successful immunological intervention was developed using therapeutic serum derived from animals immunized against diphtheria toxin. Passive immunization today involves the infusion of antigen-specific mAbs or polyclonal antibodies derived from non-human or human blood products, with convalescent plasma therapy being effective and safe with careful screening. CPT has been used to treat infections with influenza, RSV, Ebola virus, and other corona viruses. However, in rare cases, pathogen-specific antibodies can augment virulence through a process called antibody-dependent enhancement (ADE) as shown in the figure below.
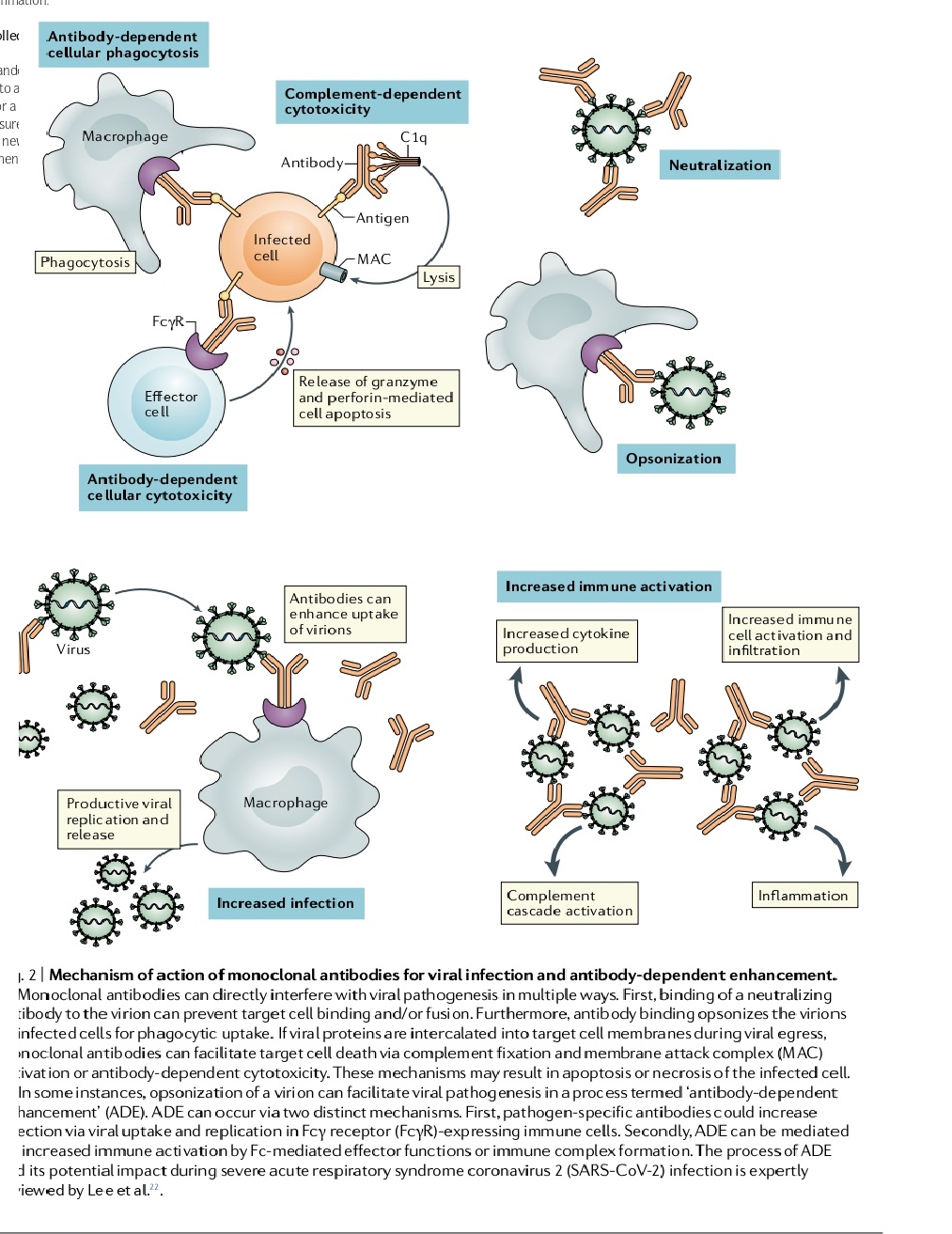
Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC)
ADCC is a process by which antibodies attach to target cells and recruit effector cells to induce target cell death. IgG1 is the most commonly used subclass of human antibodies for cancer therapeutic antibodies. Effector cells must express Fc receptors (FcR) to carry out ADCC, and Fcγ R is the most important class of FcR for tumor cell clearance by myeloid cells. Effector cells include NK cells, monocytes, macrophages, neutrophils, eosinophils, and dendritic cells. These effector cells mediate target cell death through three key mechanisms: cytotoxic granule release, Fas signaling, and elaboration of reactive oxygen species. NK cells appear to be the major effector cell type in vivo for cancer immunotherapy. NK cells highly express activating Fcγ RIIIA and do not express the inhibitory Fcγ RIIB, making antibody interactions specifically through Fcγ RIIIA of interest for cancer immunotherapy.
Complement-Dependent Cytotoxicity (CDC)
CDC is an immune reaction that destroys targeted cells by activating and attracting the complement cascade to the surface of those cells. MAbs targeting certain proteins on B cells are effective drugs for chronic lymphocytic leukemia (CLL). Alemtuzumab (ALM) is a CD52-specific mAb that works well for CLL, particularly in patients with TP53-defective or purine analog refractory disease. Rituximab (RTX) is a CD20-specific mAb that has significantly increased response rates in CLL treatment, and the addition of RTX to other drugs has improved overall survival. Ofatumumab (OFA), a human anti-CD20 mAb recently approved by the FDA, is also effective for CLL. However, despite their efficacy, we still do not fully understand how these mAbs work or why CLL cells can become resistant to them. Possible mechanisms of mAb-induced cytotoxicity include complement-dependent cytotoxicity (CDC), cell-mediated cytotoxicity, and direct induction of cell death. Studies have shown that CDC is important for ALM and OFA but not for RTX, and that antibody-dependent cellular cytotoxicity (ADCC) may play a role in the mechanism of action of all three mAbs. However, the relative importance of each mechanism in the treatment of CLL remains unclear.
Immune Checkpoint Blockade
Immune checkpoint blockade is a type of immunotherapy that involves the use of drugs to block certain proteins on immune cells, called checkpoint inhibitors that prevent the immune system from attacking cancer cells. By blocking these proteins, the therapy helps to unleash the immune system’s ability to recognize and destroy cancer cells. This approach has shown promising results in treating several types of cancer.
Checkpoint inhibition is a novel immunotherapy strategy that can transform the treatment of various types of cancer. It works by altering the body’s natural regulation of T cell function. The FDA has approved Ipilimumab, the first checkpoint inhibitor, for the treatment of metastatic melanoma. It blocks the inhibitory signaling of the CTLA-4 molecule found in T cells. Since this mechanism is not specific to one tumor type, Ipilimumab is also being studied for the treatment of other cancers, such as breast, lung, prostate, and renal cancer. The second generation of checkpoint inhibitors, including PD-1 and PD-L1 inhibitors, also show promise in clinical trials.
Redirected T-cell Killing
CD3 bispecific antibodies have the potential for treating various cancers by redirecting T cells to attack tumor cells. They work by linking the CD3 component of the T cell receptor complex with a tumor-associated antigen on the target cell’s surface, regardless of HLA restriction. This makes them useful for low neoantigen content and low inflammation tumors and can be combined with checkpoint blockade. Many formats and optimization approaches have been developed, and clinical trials have shown promising therapeutic activity. Therefore, CD3 specifics could be a valuable addition to cancer treatment.
Monoclonal antibodies have become an important class of drugs for treating various types of cancer. Several unconjugated antibodies and radioimmunoconjugates targeting CD20 have shown significant antitumor activity in both indolent and aggressive B-cell non-Hodgkin lymphoma. A CD33 antibody conjugated with calicheamicin has been approved for refractory acute myeloid leukemia, while immunotoxins targeting CD22 have demonstrated antitumor activity in hairy cell leukemia. An unconjugated antibody targeting HER2/neu is widely used alone or with chemotherapy in breast cancer, improving relapse-free survival as adjuvant therapy for HER2/neu-expressing breast cancer. An unconjugated antibody targeting vascular endothelial growth factor improves survival in metastatic colorectal cancer. Antibodies targeting B-cell idiotype and CD20 are useful for treating lymphomas. CD52 antibody fixing complement is approved for chemotherapy-refractory chronic lymphocytic leukemia. Antibodies targeting the extracellular domain of the epidermal growth factor receptor are clinically active in advanced colorectal cancer. Additionally, antibodies that block the function of the CTLA-4 co-receptor on T cells have shown promise in enhancing host immune responses to self-tumor antigens in preclinical and clinical studies.
References
- Renn, A., Fu, Y., Hu, X., Hall, M. D. & Simeonov, A,” Fruitful neutralizing antibody pipeline brings hope to defeat SARS-Cov-2”, Trends Pharmacol. Sci. 41, 815–829 (2020).
- Shanmugaraj, B., Siriwattananon, K., Wangkanont, K. & Phoolcharoen, W. “Perspectives on monoclonal antibody therapy as a potential therapeutic intervention for coronavirus disease-19 (COVID-19)”. Asian Pac. J. Allergy Immunol. 38, 10–18 (2020).
- Cheng, Y. et al. “Use of convalescent plasma therapy in SARS patients in Hong Kong”. Eur. J. Clin. Microbiol. Infect. Dis. 24, 44–46 (2005).
- Taylor, P.C., Adams, A.C., Hufford, M.M., et al.” Neutralizing monoclonal antibodies for the treatment of COVID-19”. Nat Rev Immunol 21, 382–393 (2021). https://doi.org/10.1038/s41577-021-00542-x.
- Nisar A. Baig, Ronald P. et. Al, “Complement dependent cytotoxicity in chronic lymphocytic leukemia: ofatumumab enhances alemtuzumab complement-dependent cytotoxicity and reveals cells resistant to activated complement”, Pages 2218-2227, 2012, https://doi.org/10.3109/10428194.2012.681657.



