Factors affecting ELISA in terms of Reliability
The ability to consistently produce accurate results refers to reliability in Elisa test. When the same results are produced multiple times with the same sample under the same conditions it is a reliable enzyme-linked immunosorbent assay (ELISA) test.
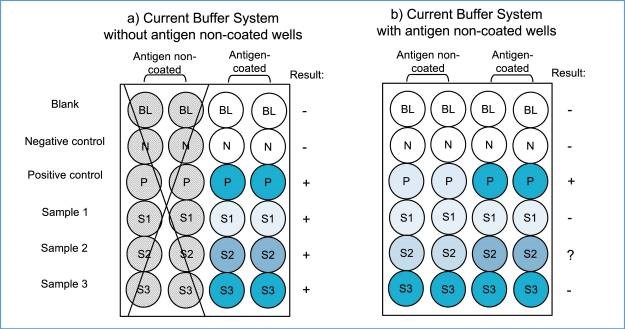
It is important to consider the principle of the immunoassay system and all types of non-specific reactions involved to prevent further misuse of the ELISA technique and misinterpretation of serological antibody assay data. In antibody assays using indirect ELISA, false positive and negative reactions can occur due to various reasons, not related to the antigens. Among these, the most intense false positive reaction is caused by BG noise reaction, resulting from the hydrophobic binding of immunoglobulin components in the sample specimens to solid surfaces. This reaction is unique to each sample and can vary significantly, sometimes even surpassing the true antibody-antigen reaction. The BG noise reaction can be easily identified by analyzing the OD values in antigen-non-coated wells and comparing them to the values obtained in antigen-coated wells. Unfortunately, this step is often overlooked and the OD values are determined solely in antigen-coated wells.
ELISA test can be affected by various factors in terms of reliability, including:
- Quality of the reagents: antibodies, enzymes, and substrates
- Contamination of the samples or reagents
- Time duration and incubation temperature during the process
- Degradation and loss of activity due to improper handling and storage of reagents
Factors affecting ELISA in terms of Accuracy
- The ability to correctly identify the presence or absence of the target antigen refers to the accuracy of ELISA test. The accuracy of traditional ELISAs in detecting leprosy varies depending on the type of leprosy, with good accuracy in detecting MB leprosy and poor accuracy in detecting PB leprosy. The WHO has identified the need for new tools to aid in early detection, and a standardized assay that can be used across regions with different epidemiological profiles and pathogen strains is necessary to achieve this goal. These laboratory tools are expected to become crucial in the future for diagnosing both types of leprosy, monitoring household contacts, and informing public health interventions.
- Quality of reagents used in assay including antigen, antibodies and enzymes affect the accuracy. If poor quality is used it can produce false positive or false negative test.
- During sample preparation errors such as sample collection, storage, handling and processing, contamination can interfere accuracy of the test results.
- Cross-reactivity occurs when the test detects antibodies or antigens that are similar to, but not identical to, the target antigen or antibody.
- Drugs or natural compounds interferences during the binding of antigens or antibodies
- The sensitivity refers to its ability to detect small amounts of the target antigen or antibody. Low sensitivity tests may produce false negative results, while high sensitivity test may produce false positive results.
- The specificity refers to its ability to detect only the target antigen or antibody and not other related substances.
- Operator error by not following manufacturer’s instructions
- High background noise, inconsistent results and low signal intensity can be minimized by choosing appropriate sample preparations and selecting appropriate reagent preparations.
Sample preparation
- It is important to select appropriate methods that can be based on the sample type and desired assay outcome. Common factors may arise during the preparation of sample like interference from matrix components or nonspecific binding of the analyte.
- Sample quality, assay conditions (Temperature and pH), incubation time and reagent quality can affect accuracy.
- Recent ELISA technology such as Matrix enzyme-linked immunosorbent assay (ELISA) and digital enzyme-linked immunosorbent assay (ELISA) can improve the accuracy and reliability of cytokine detection.
- The various factors which can cause interference like hemolysis, medication use and lipemis can be minimized by proper sample handling and preparation and the use of interference-resistant assay formats.
- Factors that can interfere with appropriate ELISA testing can occur at any phase of the testing process, beginning with specimen collection. The quality and integrity of the assay plate, coating buffer, capture antibody, blocking buffer, target antigen, detection antibody, enzyme conjugate, washes, substrate, signal detection can all interfere with proper ELISA testing.
Assay optimization
Optimization involves identifying and minimizing sources of variability in the assay, such as variation in reagent concentrations or incubation times. Factors that can affect assay optimization include identifying and minimizing sources of variability in the assay like reagents concentration variations. It can be achieved by appropriate controls and testing different combinations of reagents and conditions. Systematic approach, controls and validation with choice of antibodies, enzyme substrate system and reaction buffer pH used can improve the accuracy of ELISA tests.
Various strategies can be employed to optimize ELISA procedures, including the use of appropriate blocking buffers, optimizing antigen concentration and detection antibody concentration, and adjusting incubation times and temperatures. It is important to select reagents (enzyme and substrate) that ensure optimization of the signal-to-noise ratio and dynamic range of the assay.
DoE is a powerful tool that is gaining success in the optimization of any process that is affected by multiple factors.
The need for standardization of ELISA procedures must be ensured for consistency.
Data analysis
Accuracy and reliability can be based on the selection of appropriate data normalization method, identification and correction of outliers and proper statistical data analysis. The data generated by ELISA tests are often complex and require careful analysis to avoid errors or biases.
It is necessary to account for differences in the initial sample concentration and variations in the assay conditions for the normalization of data. Several normalization methods are available, including the use of internal standards, positive controls, and sample dilution factors. Its choice should be based on the specific experimental design and assay requirements.
Outliers can arise due to technical errors or biological variability and can be identified using Grubb’s test removed and corrected using interpolation or substation with a value within the expected range.
The choice of statistical method should be based on the design of experiment, data collection type, and the research question being addressed. Common statistical methods used in ELISA data analysis include t-tests, ANOVA, regression analysis, and correlation analysis.
ELISA test results are accurate and reliable based on appropriate data analysis. It is essential to follow good laboratory practices, including proper documentation and data management, to ensure the integrity and reproducibility of the data.
Testing different approaches to analyzing ELISA data, including using raw optical density (OD) values, normalized OD values, and dichotomized (positive/negative) results were studied. The authors compared the sensitivity and specificity of each approach using receiver operating characteristic (ROC) curves and area under the curve (AUC) analysis.
Normalization of the OD values improved the accuracy and reliability of the results. They recommend the use of non-parametric statistical tests, such as the Mann-Whitney U test, to evaluate differences between groups and to control for inter-assay variability. The use of normalization techniques such as log-transformations or standardization of samples to further control for inter-assay variability was recommended.
References
1. “An ELISA protocol to improve the accuracy and reliability of serological antibody assays”, Takaki Waritani, Jessica Chang, Bonnie McKinney, and Kuniaki Terato MethodsX. 2017; 4: 153–165, Published online 2017 Mar 30. doi: 10.1016/j.mex.2017.03.002.
2.“Accuracy of Enzyme-Linked Immunosorbent Assays (ELISAs) in Detecting Antibodies against Mycobacterium leprae in Leprosy Patients: A Systematic Review and Meta-Analysis”, Omar Ariel Espinosa, Silvana Margarida Benevides Ferreira, Fabiana Gulin Longhi Palacio, Denise da Costa Boamorte Cortela, and Eliane Ignotti. Review Article | Open Access Volume 2018 | Article ID 9828023 | https://doi.org/10.1155/2018/9828023.
3. Aydin S.,“A short history, principles, and types of ELISA, and our laboratory experience with peptide/protein analyses using ELISA”, Peptides. 2015 Oct; 72: 4-15. [PubMed]
4. Kohl TO, Ascoli CA. Indirect Immunometric ELISA. Cold Spring Harb Protoc. 2017 May 01; 2017(5) [PubMed] [Reference list]
5. A General Guide for the Optimization of Enzyme Assay Conditions Using the Design of Experiments Approach, Favour Chinyere Onyeogaziri, Christos Papaneophytou, SLAS Discovery Volume 24, Issue 5, June 2019, Pages 587-596.
6. Sensitivity, Specificity, Receiver-Operating Characteristic (ROC) Curves, and Likelihood Ratios: Communicating the Performance of Diagnostic Tests, Christopher M Florkowski, Clin Biochem Rev. 2008 Aug; 29(Suppl 1): S83–S87.



