Since around 1990, genomic research has been receiving attention in cancer care. The Precision Medicine Initiative for cancer care was proposed in January 2015 by former US President Obama during his State of the Union address, which shifted attention towards precision medicine. This approach involves classifying cancer patients based on their oncogenic driver mutations and using molecular-targeted drugs that best match each mutation for treatment, resulting in significant improvement in disease management. Despite the number of studies suggesting a good correlation between cancer and autoimmune disorders, the exact mechanisms and pathophysiology underlying this connection are unclear. The lack of this understanding is a barrier to developing effective methods for the prevention and treatment of these diseases.
Early and accurate diagnosis of human diseases, along with targeted treatment and response evaluation, is a critical component of precision medicine. However, cancers are often heterogeneous and their molecular makeup changes over time, from disease onset to progression and during treatment. The antibody-based molecular imaging helps identify varying degrees of biomarker expression and in the identification of high-risk patients. However, antibody-based radioimmunotherapy (RIT) and photoimmunotherapy (PIT) have the potential to stop or even eradicate certain forms of cancer.
Antibody-Based Therapies: Targeting Specific Molecules and Cells for Disease Treatment
In the early 1900s, Paul Ehrlich proposed the use of antitoxins, which were later known as antibodies, as ‘magic bullets’ to cure infections and cancer. The development of monoclonal antibodies by Milstein and Köhler in 1975 generated enthusiasm for their clinical use. Nonetheless, the production of reactive antibodies, also known as human anti-animal antibodies, and the risk of allergic reactions in humans from non-human derived antibodies posed significant obstacles and impeded progress in the field.
Antibody-based treatments have the objective of pinpointing precise molecules or cells in the body, including cancerous or autoimmune disease-triggering cells. Antibodies can be tailored to target particular antigens and can be utilized in creating therapeutic agents that can either stimulate or block specific signaling pathways.
A benefit of antibody-based treatments is their ability to specifically target and affect diseased tissues while minimizing negative effects on healthy tissues. This differs from traditional chemotherapy or radiation therapies, which cause harm to both cancerous and healthy cells in the body.
Although antibody-based treatments have proven to be effective in treating certain forms of cancer, the insufficient delivery of therapeutic antibodies to certain tissues can limit their effectiveness. Therefore, improving the specificity of antibodies for both antigens and tissues can enhance the efficacy of antibody therapy in the clinical setting.
Several categories of therapies that use antibodies are available, including monoclonal antibodies, bispecific antibodies, antibody-drug conjugates, and immune checkpoint inhibitors.
Monoclonal Antibodies (mAbs) are designed to bind to a specific antigen, which can either be a protein, a carbohydrate, or a lipid. Large quantities of these antibodies can be generated and utilized to hinder the function of particular proteins or to transport drugs to cancerous cells.
Behring and his colleagues may have been the first to use antibody-based therapy in 1890. However, it wasn’t until 1975 that the production of identical antibodies that specifically target a particular antigen became possible. This resulted in the development of therapeutic monoclonal antibodies (mAbs). Despite their specificity for targets, the usefulness of murine mAbs as human therapeutics was limited due to two major issues. First, they didn’t always activate human effector functions, despite their target specificity. Second, murine antibodies were recognized by the human immune system and caused the production of human anti-mouse antibodies (HAMA response).
More than 150 clinical trials are being conducted worldwide to test monoclonal antibodies, which are presently the most extensively examined recombinant proteins. They also make up around 25% of new biotech drugs currently under development. In 2006, seven monoclonal antibody products that were authorized for clinical use earned more than US$1 billion in global sales. Among these products, infliximab and adalimumab, which are employed for treating autoimmune disorders, as well as rituximab, which is utilized to treat non-Hodgkin’s lymphoma, B-cell leukemia, and autoimmune disorders, had sales surpassing US$2 billion each.
Bispecific Antibodies (BsAbs) are a type of antibodies created to identify and attach to two distinct antigens simultaneously. They can be utilized to connect cancer cells with immune cells, leading to the eradication of cancer cells. BsAbs can recognize two distinct epitopes. This unique feature allows for a wide range of potential applications, such as redirecting T cells to target tumor cells, blocking two separate signaling pathways simultaneously, dual targeting of different disease mediators, and delivering therapeutic payloads to specific sites. The approval of catumaxomab, which targets both EpCAM and CD3, and blinatumomab, which targets both CD19 and CD3, has been a significant breakthrough in the advancement of bispecific antibodies.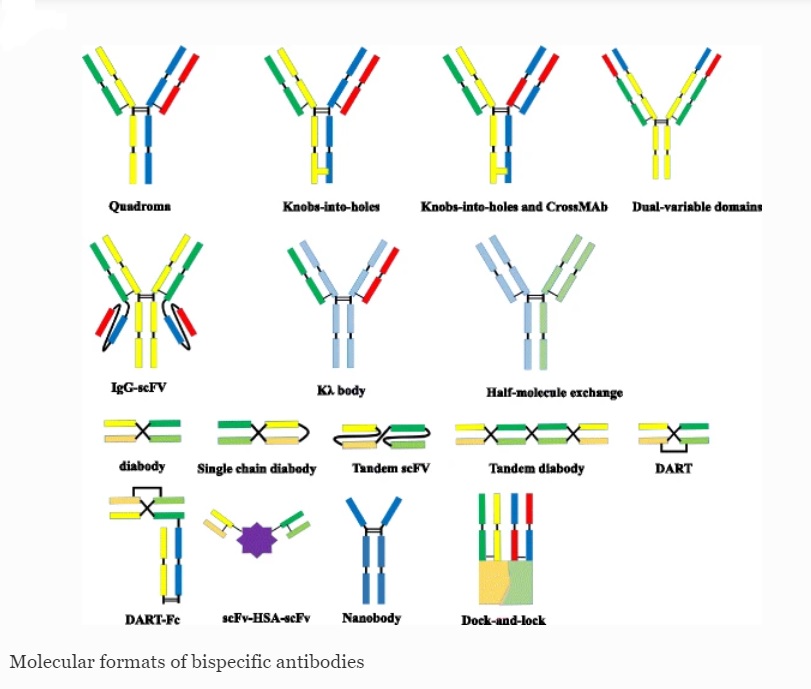
The production of bsAbs has faced challenges such as product instability, low expression yields, and immunogenicity, which have hindered their development. However, advancements in the design of newer bsAbs formats have made them more stable, easier to produce, and less immunogenic. Despite this, immunogenicity remains a complex issue in drug development, and adverse effects during clinical trials often hinder their success. These adverse effects are mainly caused by a “cytokine storm.” The progress made in the development of bsAbs gives hope for the availability and approval of more therapeutic alternatives in the future. Currently, there are over 60 different formats of BsAbs available, with some already progressing through clinical trials.
Antibody-Drug Conjugates (ADCs). The concept of ADCs involves the combination of monoclonal antibodies and cytotoxic drugs in a single molecular entity, providing a new class of cancer treatments that utilize the targeting advantages of mAbs and the cytotoxic potential of small molecules to achieve specific drug delivery to tumor cells through the interaction between the antibody and antigen. The delivery of chemotherapy drug directly to the cancer cells helps minimize damage to healthy cells. However, selecting an appropriate target antigen is crucial for the successful development of ADCs. The selection process involves several factors to consider, including high expression levels of the target antigen in tumors, limited or no expression in normal tissues, or expression limited to a particular tissue type, to reduce off-target toxicity and provide a suitable therapeutic index for the ADC.
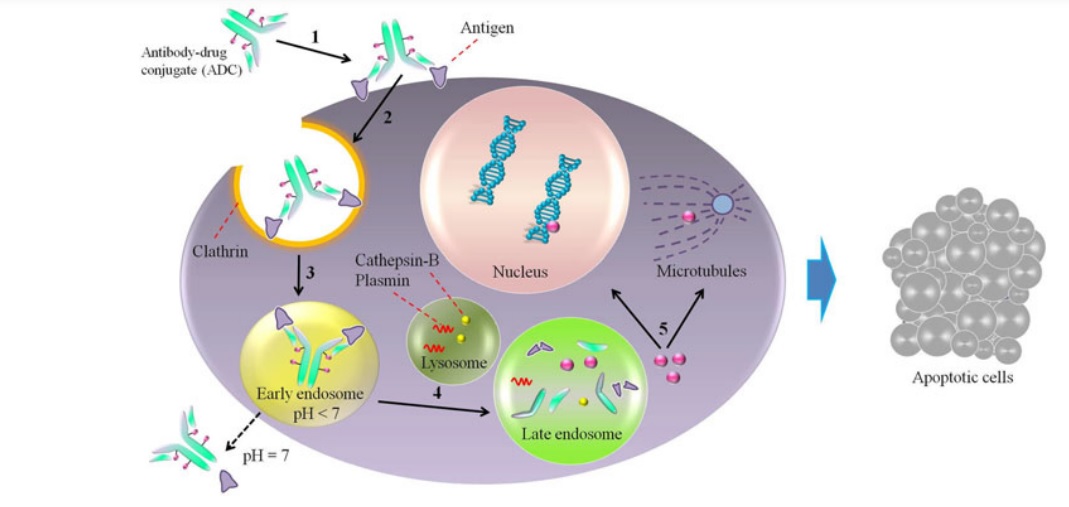
The design of improved compounds for antibody-drug conjugates (ADCs) has been guided by lessons learned from first-generation ADCs and advancements in technology. This has resulted in a dynamic ADC field with over 60 ADCs currently in clinical trials, including more than 30 new ones since 2013. The only marketed ADC is brentuximab vedotin, which consists of an anti-CD30 antibody conjugated to a potent microtubule inhibitor used to treat Hodgkin’s lymphoma and anaplastic large cell lymphomas. T-DM1, an ADC composed of trastuzumab conjugated to DM1 via a non-cleavable linker, is also showing promising results in phase III for HER2-positive refractory/relapsed metastatic breast cancer.
Immune Checkpoint Inhibitors have revolutionized the treatment of several types of cancers, including melanoma, lung cancer, and kidney cancer. To avoid autoimmune disorders, the immune system has an innate mechanism that prevents the over-stimulation of T cells. This mechanism is controlled by proteins known as immune checkpoints, which can suppress or activate T cells. T cells are the immune cells that recognize and kill cancer cells. Two main types of immune checkpoint inhibitors that are currently used in clinical practice are: PD-1 inhibitors and CTLA-4 inhibitors.
The discoveries of PD1 and PD-L1 have led to the revolution of modern cancer immunotherapy. PD-1 inhibitors block the activity of the programmed cell death protein 1 (PD-1), which is an immune checkpoint protein found on the surface of T cells. PD-1 binds to its ligands, PD-L1 and PD-L2, which are expressed on the surface of cancer cells and other cells in the tumor microenvironment. This interaction suppresses the activity of T cells, allowing the cancer cells to evade detection and destruction by the immune system. PD-1 inhibitors such as pembrolizumab and nivolumab can block this interaction, leading to the activation of T cells and the destruction of cancer cells.
CTLA-4 inhibitors block the activity of the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4). CTLA-4 competes with the co-stimulatory molecule CD28 for binding to the antigen-presenting cells (APCs) during T cell activation. By doing so, CTLA-4 inhibits the activation of T cells. CTLA-4 inhibitors such as ipilimumab can block this interaction, leading to the activation of T cells and the destruction of cancer cells.
Benefits of Combining Antibody-Based Therapies and Precision Medicine
Combining antibody-based therapies with precision medicine can offer several benefits for the treatment of various diseases. Precision medicine involves identifying the unique genetic, molecular, and environmental factors that contribute to a patient’s disease, which can guide personalized treatment decisions. Antibody-based therapies, on the other hand, are targeted therapies that can selectively bind to specific antigens or proteins that are overexpressed or aberrantly expressed in the disease state. When these two approaches are combined, it can lead to better outcomes for patients.
Here are some of the benefits of combining antibody-based therapies with precision medicine:
- Enhanced Efficacy: Precision medicine can help identify patients who are most likely to respond to a particular antibody-based therapy. For example, in cancer treatment, a genetic test can identify the presence of specific mutations that make the cancer cells vulnerable to a particular antibody-based therapy.
- Reduced Side Effects: Antibody-based therapies are highly specific and targeted, which means that they can potentially spare normal healthy cells from damage. Precision medicine can help identify patients who are less likely to experience adverse effects from the therapy, which can minimize the risk of side effects and improve the quality of life of patients.
- Overcoming Resistance: In some cases, cancer cells can develop resistance to antibody-based therapies over time. Precision medicine can help identify the underlying molecular mechanisms that drive resistance and guide the selection of a different therapy or combination of therapies that can overcome resistance.
- Combinatorial Approaches: Combining multiple antibody-based therapies or combining antibody-based therapies with other treatment modalities, such as chemotherapy or radiation therapy, can lead to better outcomes for patients.
- Tailored Clinical Trials: Precision medicine can also help identify patient populations that are most likely to benefit from a particular antibody-based therapy, which can guide the design of clinical trials. This can lead to more efficient and effective clinical trials and accelerate the development of new therapies.
References
- Miyagawa I and Tanaka Y (2022) Dawn of Precision Medicine in Psoriatic Arthritis. Med.9:851892. doi: 10.3389/fmed.2022.851892
- Tian Z, Wu L, Yu C, Chen Y, Xu Z, Bado I, Loredo A, Wang L, Wang H, Wu KL, Zhang W, Zhang XH, Xiao H. Harnessing the power of antibodies to fight bone metastasis. Sci Adv. 2021 Jun 23;7(26):eabf2051. doi: 10.1126/sciadv.abf2051. PMID: 34162538; PMCID: PMC8221630.
- Antibody-Based Imaging and Therapy for Precision Medicine Weijun Wei, Dawei Jiang, Laura Evangelista, and Weibo CaizMolecular Pharmaceutics2022 19 (10), 3453-3455 DOI: 10.1021/acs.molpharmaceut.2c00606
- Masahiro Yasunaga, Antibody therapeutics and immunoregulation in cancer and autoimmune disease, Seminars in Cancer Biology, Volume 64, 2020, Pages 1-12, ISSN 1044-579X, https://doi.org/10.1016/j.semcancer.2019.06.001.
- Fan, G., Wang, Z., Hao, M. et al. Bispecific antibodies and their applications. J Hematol Oncol 8, 130 (2015). https://doi.org/10.1186/s13045-015-0227-0
- Ducry, Laurent (2013). [Methods in Molecular Biology] Antibody-Drug Conjugates Volume 1045 || Antibody–Drug Conjugate (ADC) Clinical Pipeline: A Review. , 10.1007/978-1-62703-541-5(Chapter 1), 1–27. doi:10.1007/978-1-62703-541-5_1
- Abdollahpour-Alitappeh, Meghdad; Lotfinia, Majid; Gharibi, Tohid; Mardaneh, Jalal; Farhadihosseinabadi, Behrouz; Larki, Pegah; Faghfourian, Babak; Sepehr, Koushan Sineh; Abbaszadeh-Goudarzi, Kazem; Abbaszadeh-Goudarzi, Ghasem; Johari, Behrooz; Zali, Mohammad Reza; Bagheri, Nader (2018). Antibody-drug conjugates (ADCs) for cancer therapy: Strategies, challenges, and successes. Journal of Cellular Physiology, (), –. doi:10.1002/jcp.27419
- The discoveries of PD1 and PD-L1 have led to the revolution of modern cancer immunotherapy [51]. Multiple agents targeting PD1, PD-L1, or CTLA-4 either as single agent or combination regimens are widely used as ICIs which alleviate the suppression of immune regulatory machineries and lead to immunoablation of once highly refractory cancer cells [52,53,54,55]. Recent discoveries on the immunomodulatory effects of gut microbiota shed lights on new ways in enhancing cancer immunotherapy [56].