The Centers for Medicare & Medicaid Services (CMS) established quality standards for clinical laboratories that are required to report patient-specific test results for diagnosis, prevention, treatment, or assessment of human health. These CLIA standards ensure the validity of laboratory test results and protect patients. However, research laboratories often lack comparable quality-assurance processes, which can make it difficult to assess the validity of individual test results. The absence of a minimum quality management system requirement for research laboratories, like the CLIA standards for clinical laboratories, creates uncertainty concerning the validity of test results. Therefore, compliance with CLIA standards is essential to ensure accurate and reliable testing results, as well as protect patient safety.
Importance of Quality Processes in Scientific Research Testing
Experts recommend high validity for individual research results to be returned to participants, which depends on the test used and the laboratory environment. However, the quality management systems in place in different research settings vary, making it challenging to determine the validity of research results. This lack of certainty is a barrier to returning research results. Therefore, appropriate quality processes in scientific research are essential for ensuring result validity and maintaining the quality of science.
The entire process of research testing involves the pre-analysis, analysis, and post-analysis phases. This process begins with the formulation of a research question, selection of appropriate tests by the investigator, sample collection, transport, and processing, followed by analysis, interpretation, and potential impact on new hypotheses or participant care. To prevent errors in the analytical and post-analytical phases, strict measures should be taken during the pre-analytical phase to avoid mistakes associated with specimen handling and identification.
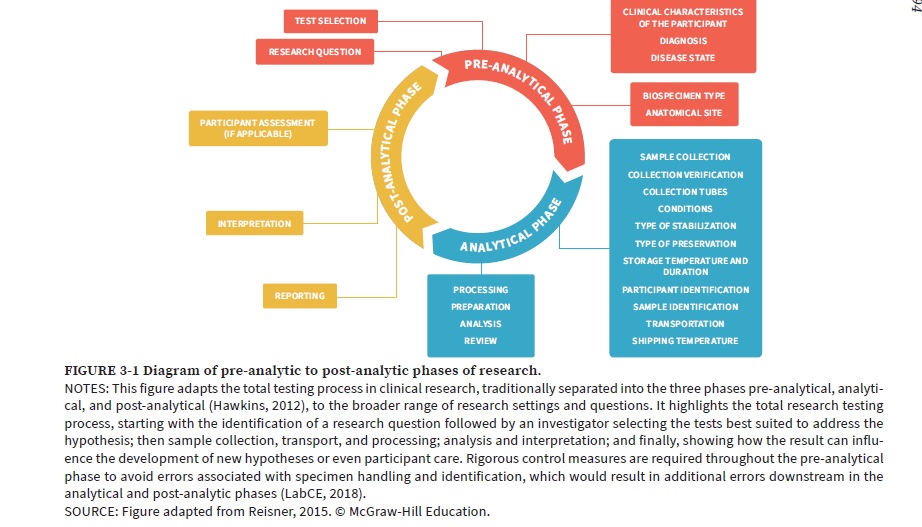
Biospecimen Research Phases and Quality Processes
Human Biospecimen translational research includes a range of phases, from basic research to clinical trials. The laboratory methods and quality processes vary depending on the research phase. Basic research needs flexible procedures, while clinical research requires more regulated quality management systems. The closer the research is to clinical application, the more likely individual research results can be returned, but it depends on the study’s level of investigator-participant interaction. Longitudinal studies have more interactions with participants than environmental exposure studies.
The Need for Quality Standards in Research Laboratories
Research laboratories that are not CLIA-certified can still maintain high-quality standards that exceed CLIA requirements. Some laboratories, such as the Clinical Sequencing Evidence-Generating Research (CSER) consortium, have highly automated processes that are less prone to error than CLIA-certified laboratories. However, other research laboratories may not have established internal operational standards or meet any formally recognized quality standards, which limit the ability of investigators to demonstrate the validity of their results.
Importance of Quality Practices
In addition to assessing laboratory quality, IRBs (The Institutional Review Board) must consider other factors when deciding whether to return research results to participants, including the value of the information and the potential risks involved. If researchers do not adhere to quality practices, returning results is not recommended. However, with proper oversight and a research quality management system (QMS) in place, returning results is appropriate. The committee recommends the development of a research QMS to ensure the validity of results and provide ongoing control processes. However, substantial investment in infrastructure will be necessary to increase the number of laboratories meeting quality standards for returning results. Researchers will need guidance and support from institutions and sponsors. The initial cost and time commitment may be high, but the value to participants and biomedical research will be significant. Although the difficulties associated with implementing a QMS can be considerable, the importance of ensuring quality is also significant.

Implementing Effective Quality Control Measures
Here are some of the quality control measures that are part of CLIA compliance:
Quality Control (QC) Program: CLIA requires laboratories to establish and maintain a QC program that includes testing controls, calibrators, and reference materials to ensure the accuracy and precision of test results.
Proficiency Testing (PT): Laboratories must participate in PT programs to evaluate their performance and ensure that their test results are accurate and reliable.
Personnel Competency Assessment: CLIA requires laboratories to ensure that their personnel is competent and qualified to perform the tests they are assigned to. To maintain staff proficiency in a laboratory, it is necessary to establish clear responsibilities, provide appropriate training, conduct competency assessments, document compliance with policies, implement risk mitigation strategies, and continuously review and update training programs.
Standard Operating Procedures (SOPs): Laboratories must develop and implement written SOPs for all laboratory procedures, including specimen collection, handling, and processing, as well as test performance, interpretation, and reporting. To maintain proper laboratory infrastructure, it is essential to validate equipment and implement procedures for change control, calibration, maintenance, repair, and environmental monitoring, including temperature monitoring of freezers. Additionally, a supplier management program should be established to inspect and validate reagents and supplies used in the laboratory.
Quality Assessment: CLIA requires laboratories to perform ongoing quality assessment activities to identify and correct problems that may affect the accuracy and reliability of test results.
Quality Control Records: To ensure proper recordkeeping and document control in a laboratory, the following measures should be taken: implement a system for managing data quality, assessment, and reporting; maintain clinical data records; ensure policies and procedures are accessible; keep documentation records such as audit reports, deviation reports, and corrective action/preventive action reports; conduct external monitoring to ensure compliance and maintain staff training and supply records.
Internal Audits Ensure Accuracy and Integrity of Biospecimen Resource
An internal audit of the program and its policies is necessary to ensure the accuracy of annotation data and patient information associated with biospecimens. The audit also checks for compliance with institution policies on human subjects, privacy, and confidentiality. Additionally, standard operating procedures for all activities and processes are reviewed and approved, with a process in place for regular review and updating. Overall, these audits help maintain the integrity of the bio specimen resource.
Techniques for Monitoring and Evaluating Quality Control Measures
To evaluate the effectiveness of quality control measures in CLIA, various techniques can be utilized including Internal Quality Control, External Quality Control, Quality Assurance, Corrective and Preventive Action, and Data Analysis. These methods help to ensure that laboratory testing meets the highest standards for patient care by identifying discrepancies, addressing issues, and continuously monitoring and improving the entire testing process.
Metrics for Evaluating Quality Control in Clinical Labs
When evaluating and monitoring quality control measures in a clinical laboratory, it is important to ensure that the metrics being used align with the goals of the laboratory and the specific processes being monitored. Some commonly used metrics for CLIA quality control include defect rate, first-time pass rate, customer satisfaction, cycle time, and cost of quality, process capability, and scrap or rework rate. A combination of these metrics should be used to track performance over time and identify areas for improvement in the laboratory’s quality control process.
References
- Yost, J. A. 2003. Laboratory inspection: The view from CMS. Laboratory Medicine 34(2):136–140.
- N. Baker, M. O’Leary, G. Reaman, P. C. Adamson, and S. Joffe. 2012. Recommendations for the return of research results to study participants and guardians: A report from the Children’s Oncology Group. Journal of Clinical Oncology 30(36):4573–4579.
- Bookman, E. B., A. A. Langehorne, J. H. Eckfeldt, K. C. Glass, G. P. Jarvik, M. Klag, G. Koski, A. Motulsky, B. Wilfond, T. A. Manolio, R. R. Fabsitz, and R. V. Luepker. 2006. Reporting genetic results in research studies: Summary and recommendations
- Green, R. C., J. S. Berg, W. W. Grody, S. S. Kalia, B. R. Korf, C. L. Martin, A. L. McGuire, R. L. Nussbaum, J. M. O’Daniel, K. E. Ormond, H. L. Rehm, M. S. Watson, M. S. Williams, and L. G. Biesecker. 2013. ACMG recommendations for reporting incidental findings in clinical exome and genome sequencing. Genetics in Medicine 15(7):565–574.
- Jarvik, G. P., L. M. Amendola, et.al; Electronic Medical Records and Genomics Act–Return of Results (ROR) Committee, Consent, Education, Regulation, and Consultation Committee, Clinical Sequencing Exploratory Research Act–ROR Working Group, and W. Burke. 2014. Return of genomic results to research participants: the floor, the ceiling, and the choices in between. American Journal of Human Genetics 94(6):818–826.
- Rehm, H. L., S. J. Bale, P. Bayrak-Toydemir, J. S. Berg, K. K. Brown, J. L. Deignan, M. J. Friez, B. H. Funke, M. R. Hegde, and E. Lyon. 2013. ACMG clinical laboratory standards for next-generation sequencing. Genetics in Medicine 15:733–747.



