Advancements in ELISA technology have introduced precision and innovation, significantly enhancing its sensitivity and specificity for detecting and quantifying target proteins across a variety of samples. Originally developed as an alternative to radioimmunoassay, ELISA now employs an advanced approach using capture and detection antibodies that bind specifically to antigens. This leads to measurable reactions, enabling researchers to analyze results with high accuracy. These improvements have expanded ELISA’s versatility, including the detection of hormones and other analytes in patient serum, making it a vital tool in both research and diagnostics.
One key advancement is the use of sandwich ELISA, where capture antibodies are coated onto a microplate to bind the target antigen, followed by the addition of detection antibodies. This setup minimizes cross-reactivity and ensures accurate quantification. The reaction leads to a color change, measured by absorbance, which directly correlates to the quantity of the antigen present. This approach enhances the efficiency and reliability of quantifying the amount of analyte in the sample, providing critical information even in the absence of high concentration levels.
One key advancement is the use of sandwich ELISA, where capture antibodies are coated onto a microplate to bind the target antigen, followed by the addition of detection antibodies. This setup minimizes cross-reactivity and ensures accurate quantification. The reaction leads to a color change, measured by absorbance, which directly correlates to the quantity of the antigen present.
In recent years, the emergence of pandemics has underscored the importance of ELISA in developing rapid, reliable diagnostics and monitoring therapeutic responses. The improvement in assay methods has increased the efficiency of detecting various targets and provided more detailed information about the presence and quantity of specific analytes. Scientists have also improved the assay’s flexibility, allowing for the simultaneous detection of multiple targets, further solidifying ELISA’s role in advancing modern medicine and therapeutics.
Technological Advancements in ELISA
- ELISA in Drug Screening: Advancements in ELISA make drug screening more selective and efficient for natural products, small molecules, and antibodies, offering significant advantages such as faster processing and cost reduction.
- Software for bELISA: The addition of advances software like Adobe Illustrator, Origin, and SPSS help improve bio-affinity ELISA methods by helping with assay design, data analysis, and increasing the value of diagnostics.
- Co-ELISA vs. Cl-ELISA: Co-ELISA and Cl-ELISA techniques, for example, are used to identify specific agents like Imidacloprid (IMI) in vegetables. Co-ELISA has a wide detection range while Cl-ELISA is more sensitive and cost-effective. The variety of detection ranges and sensitivity levels helps select the most suitable method, demonstrating ELISA’s potential for diverse applications.

- NULISA™: NULISA™ enhances traditional ELISA by detecting very low levels of biomarkers using DNA-linked antibodies and next-gen sequencing, enhancing the precision and potential of diagnostic assays, particularly in the detection of autoimmune diseases and COVID-19.
- BioTek Elx405: Automation and new equipment, like the BioTek ELx405, have streamlined ELISA processes, reducing variability and allowing for high-throughput screening. This is particularly valuable in the study of cytokines and autoantibodies in conditions like Multisystem Inflammatory Syndrome in Children (MIS-C) and COVID-19.
Nanotechnology Integration in ELISA
-
Artificial Enzymes: New ELISA methods use artificial enzyme mimics, which make the tests more sensitive and stable for protein detection. These changes improve the performance of biosensing techniques and offer new potential in diagnostics.
-
Quantum Dots (QDs): QDs provide adjustable light emissions and high stability, enhancing sensitivity and allowing multiple tests at once in bio assays.
-
Nanomaterials in Immunoassays: Recombinant protein-based ELISA and immunochromatographic tests (ICT) are crucial for accurate COVID-19 detection. Using nanomaterials to boost signals in ICT makes point-of-care testing more effective.
Multiplexed ELISA Assays
Microarray ELISA:
- New microarray ELISA methods detect multiple biomarkers in a sample, which is important for Neurodegenerative disoders.
- Researchers can now analyze a broad spectrum of proteins and agents with greater precision, thanks to the high-throughput capabilities enabled by IoT, 5G-IoT, and AI technologies (Machine learning/Deep Learning). This revolutionizes personalized healthcare by making diagnostics more precise and efficient.
Group B Streptococcus (GBS) Testing:
- dLIA (Dot Blot Luminex Immunoassay) can measure specific IgG levels for six major GBS serotypes. Helps in standardized assessments for vaccines and global studies on GBS infection rates.
Label-Free Electrochemical Methods:
- Advances in detecting biomarkers for infectious diseases without needing labels. The sensitivity and specificity is enhanced through protein electrochemistry and new biosensors, making tests more accurate and reliable.
Automated and Portable ELISA Systems
MACpro Platform:
- Integration: Combines robotic microfluidic handling with multicolor sensing.
- Benefits: This equipment improves the speed, sensitivity, and automation of disease diagnosis, making it possible to perform tests outside traditional labs.
- Example: Better detection of interferon-gamma with a wider range and lower detection limits than traditional ELISA.
Evolving ELISA Technology:
- Advancements: Incorporates nanotechnology, microfluidics, and automation.
- Impact: These innovations increase the potential for ELISA to detect foodborne pathogens and contaminants which helps maintain global food safety standards.
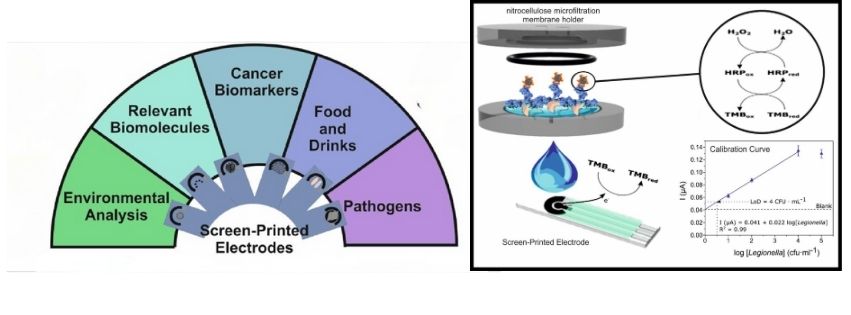
Improved Detection Methods
- Screen-Printed Electrodes (SPEs): SPEs offer a versatile approach for detecting a variety of proteins and agents in medical, environmental, and food testing. SPEs can connect with portable, smartphone-based meters, moving towards widespread commercial use.
- Surface-Enhanced Raman Scattering (SERS) Technology: SERs technology is promising for accurate pancreatic cancer diagnosis and treatment monitoring. It uses advanced probes and detects metabolites, providing real-time surgical guidance and improving clinical outcomes and treatments.
Microfluidic ELISA Assays
- Microfluidic Diagnostic Devices: Recent improvements in microfluidic devices are creating customizable, point-of-care tools that can diagnose many diseases.
- Point-of-Care HIV Testing: Using microfluidic and smartphone technologies for HIV testing provides quick, affordable results in areas with limited resources, helping to overcome infrastructure challenges.
Explore Our Full Range of ELISA Kits
Whether you’re testing human, animal, or plant samples, MyBioSource offers over 1 million ELISA kits covering thousands of analytes across every major species.
References
- Aydin S. A short history, principles, and types of ELISA, and our laboratory experience with peptide/protein analyses using ELISA. Peptides. 2015 Oct;72:4-15. doi 10.1016/j.peptides.2015.04.012. Epub 2015 Apr 20. PMID: 25908411.
- Jin, H., Cui, D., Fan, Y., Li, G., Zhong, Z., & Wang, Y. (2024). Recent advances in affinity strategies for preclinical and clinical drug discovery: Screening natural products, small molecules, and antibodies. Drug Discovery Today, 103885.
- Cao Z, Yin D, Zhang L, Ma S, Zhang K, Yang R, Shan H, Qin Z.2023.A Novel Blocking Enzyme-Linked Immunosorbent Assay Based on a Biotinylated Nanobody for the Rapid and Sensitive Clinical Detection of Classical Swine Fever Virus Antibodies. Microbiol Spectr11:e02996-22.https://doi.org/10.1128/spectrum.02996-22
- Zhai, R., Chen, G., Liu, G., Huang, X., Xu, X., Li, L., … & Abd El-Aty, A. M. (2023). Comparison of chemiluminescence enzyme immunoassay (Cl-ELISA) with colorimetric enzyme immunoassay (Co-ELISA) for imidacloprid detection in vegetables. Foods, 12(1), 196.
- Feng, W., Beer, J., Hao, Q., Ariyapala, I. S., Sahajan, A., Komarov, A., … & Ma, X. J. (2023). NULISA: a novel proteomic liquid biopsy platform with attomolar sensitivity and high multiplexing. bioRxiv, 2023-04
- Lawrence DA, Jadhav A, Mondal TK, Carson K, Lee WT, Hogan AH, Herbst KW, Michelow IC, Brimacombe M, Salazar JC, et al. Inflammatory and Autoimmune Aspects of Multisystem Inflammatory Syndrome in Children (MIS-C): A Prospective Cohort Study. Viruses. 2024;
Li, Y., Bao, Q., Wang, Z., Huang, Y., Zhang, D., Shen, Y., … & Wang, J. (2024). Artificial enzyme mimics cascade catalysis for signal amplification and transduction in food quality determination: An overview of fundamentals and recent advances. Coordination Chemistry Reviews, 505, 215689.
Farka, Z., Brandmeier, J. C., Mickert, M. J., Pastucha, M., Lacina, K., Skládal, P., … & Gorris, H. H. (2024). Nanoparticle‐based bio affinity assays: from the research laboratory to the market. Advanced Materials, 36(3), 2307653.
Parihar, A., Choudhary, N. K., & Khan, R. (2024). POCT devices for neurodegenerative disorders: from lab to clinics. In Smart Diagnostics for Neurodegenerative Disorders (pp. 279-310). Academic Press.
Furuya, Hideki, et al. “Bladder cancer risk stratification with the Oncuria 10-plex bead-based urinalysis assay using three different Luminex xMAP instrumentation platforms.” Journal of Translational Medicine 22.1 (2024): 8.
Madhurantakam, Sasya, et al. “Electrochemical label-free methods for ultrasensitive multiplex protein profiling of infectious diseases.” Current Medicinal Chemistry 31.25 (2024): 3857-3869.
Liu, Binyan, et al. “Multicolor-Assay-on-a-Chip Processed by Robotic Operation (MACpro) with Improved Diagnostic Accuracy for Field-Deployable Detection.” Analytical Chemistry 96.17 (2024): 6634-6642.
Eyvazi, S., Baradaran, B., Mokhtarzadeh, A., & de la Guardia, M. (2021). Recent advances in the development of portable biosensors for monitoring biological contaminants in foods. Trends in Food Science & Technology, 114, 712-721.
Wang, X., Zhang, Z., Wu, G., Xu, C., Wu, J., Zhang, X., & Liu, J. (2022). Applications of electrochemical biosensors based on functional antibody-modified screen-printed electrodes: a review. Analytical Methods, 14(1), 7-16.
Xu, L., Xie, Y., Lin, J., Wu, A., & Jiang, T. (2024). Advancements in SERS‐based biological detection and its application and perspectives in pancreatic cancer. View, 5(1), 20230070.
Valdez‑Cruz, Norma A., et al. “Oral administration of a recombinant modified RBD antigen of SARS-CoV-2 as a possible immunostimulant for the care of COVID-19.” Microbial Cell Factories 23.1 (2024): 41.
Rahman, N. A. A., Fuaad, A. A. H. A., Azami, N. A. M., Amin, M. C. I. M., & Azmi, F. (2024). Next-Generation Dengue Vaccines: Leveraging Peptide-Based Immunogens and Advanced Nanoparticles as Delivery Platforms. Journal of Pharmaceutical Sciences.
Lovato, Patricia Fernanda Ferreira, et al. “Feasibility of digital image colorimetric methods for iron determination in river sediment.” International Journal of Sediment Research (2024).
Steinhubl, Steven R., et al. “Wearable Sensor and Digital Twin Technology for the Development of a Personalized Digital Biomarker of Vaccine-Induced Inflammation.” medRxiv (2024): 2024-01.
Jayamohan, Harikrishnan, Himanshu J. Sant, and Bruce K. Gale. “Applications of microfluidics for molecular diagnostics.” Microfluidic Diagnostics: Methods and Protocols (2013): 305-334.



