The Clinical Laboratory Improvement Amendments (CLIA) were introduced in 1988 and revised in 2003 to establish quality standards for all laboratory testing to ensure the accuracy, reliability, and timeliness of patient test results. The underlying philosophy of CLIA is to incorporate a total quality management-based approach into the regulatory process, making laboratory directors responsible for guaranteeing appropriate quality practices are followed. The CLIA’88 regulations specify a universal set of minimum good laboratory practices that require laboratories to have trained and competent personnel, follow manufacturers’ procedural directions, perform and evaluate daily quality control, participate in external quality assessments, and document all activities. Compliance with these practices is assessed through bi-annual inspections, and laboratories that fail to comply are subject to progressive sanctions. The regulation of US laboratories has a brief history, with CLIA’88 being enacted to ensure quality in all laboratories, including physicians’ offices. CLIA recognizes three major categories of testing: waived, moderate complexity, and high complexity, with only moderate and high complexity laboratories required to participate in PT and undergo bi-annual inspections.
Compliance and Accreditation for CLIA Regulations in Laboratories
Compliance with CLIA regulations is mandatory, and laboratories must comply with federal requirements for personnel qualifications, quality control, proficiency testing, patient test management, and quality assurance. Accreditation is the voluntary process in which a laboratory can demonstrate compliance with CLIA regulations and enhance its performance, patient care, and competitiveness. Inspections and surveys are conducted to evaluate compliance with regulatory requirements, and laboratories that fail to comply may face penalties, sanctions, or revocation of certification, which can result in closure. Penalties may include fines, suspension, or limitation of laboratory operations, while sanctions can include mandatory training, increased oversight, or revocation of laboratory certification. Laboratories that receive penalties or sanctions must take corrective action to regain compliance.
Quality Standards and Accreditation Programs in Laboratories: Improving Efficiency and Reducing Errors
Adherence to quality standards and accreditation programs can improve laboratory efficiency and reduce errors. Participation in proficiency testing programs (PT) can result in more accurate test results, even after just three rounds of external PT in resource-limited settings. Accreditation may have limited effects on quality performance in high-resource settings, but it can lead to modest improvements in quality performance in Singapore’s vaginal screening laboratories. Strengthening laboratory systems for accreditation in developing country public-sector laboratories is likely to result in direct improvements in testing quality, which could ultimately benefit patients. However, more research is needed on the impact of accreditation on laboratory errors, testing quality, and patient outcomes in resource-limited settings.
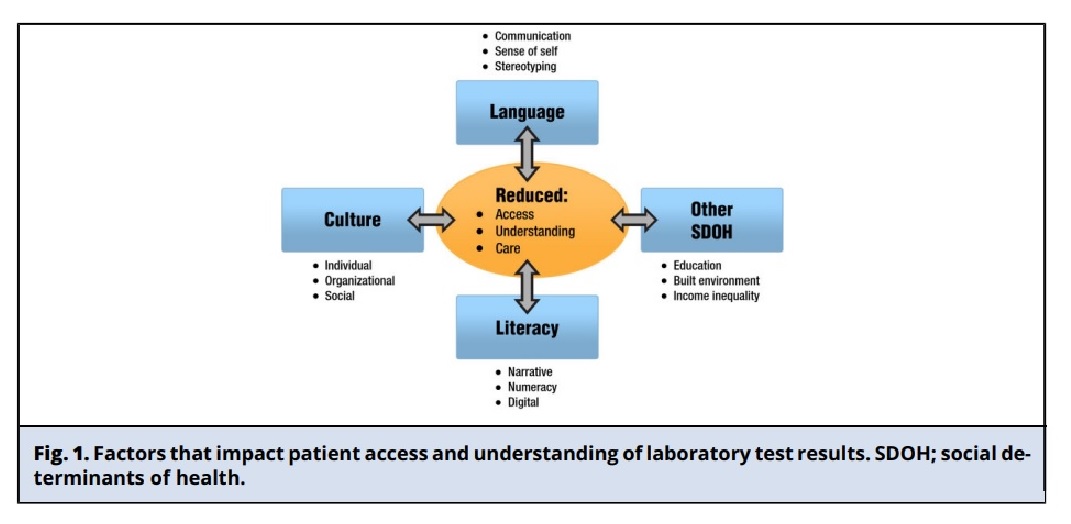
Patient Engagement and Portal Access for Vulnerable Populations
Engaging patients in reviewing their health records can improve healthcare outcomes and help identify medical errors. The federal government recommends patient engagement and shared decision-making, but vulnerable populations may face barriers to accessing patient portals, particularly those with limited health literacy or limited English proficiency. Intensive training and usability improvements are necessary to ensure meaningful access and reduce health disparities for these groups.
The AHRQ (Agency for Healthcare Research and Quality) has reported that LEP (Limited English Proficient) populations have more hospital readmissions and medical errors. Increasing access to patient portals for LEP patients may improve health outcomes and reduce health disparities.
Gaps in Making Patient Portals and Resources Available to Spanish-Speaking LEP Patients
The four key factors that contribute to the lower usage of patient portals among Spanish-speaking LEP patients compared to non-Hispanic White English-speaking patients:
Unequal access: patients with low income, education, and insurance are less likely to be offered access to patient portals or to review laboratory test results.
Technology and privacy-related barriers: Spanish-speaking LEP patients may be uncomfortable with computers and prefer in-person communication. They may also have concerns about privacy and confidentiality.
Cultural and linguistic issues: LEP patients may have negative experiences with healthcare providers due to cultural and linguistic issues such as a lack of reading abilities and misidentification errors.
Communication errors: LEP patients are at a higher risk of experiencing safety events due to communication errors, highlighting the need to implement patient safety systems for LEP patients. Healthcare organizations don’t consistently offer patient portals and resources in Spanish, which negatively affects Spanish-speaking LEP patients. This is due to concerns such as the extra time needed to answer questions, reimbursement, and technology burnout. For effective shared decision-making in a patient-centered approach, it’s crucial to engage patients, provide access, and help them understand laboratory test results.
If these gaps aren’t addressed, healthcare decisions may not improve health outcomes or achieve health equity as intended.
The gaps affecting Spanish-speaking LEP patients’ use of patient portals are interrelated and not mutually exclusive. Therefore, addressing these gaps can create opportunities to reduce disparities and improve healthcare access. By increasing the availability of resources in Spanish and providing culture-specific training, patient-provider interactions can be improved, leading to better communication, patient satisfaction, and health outcomes. Various resources and strategies can reduce disparities in Spanish-speaking LEP populations.
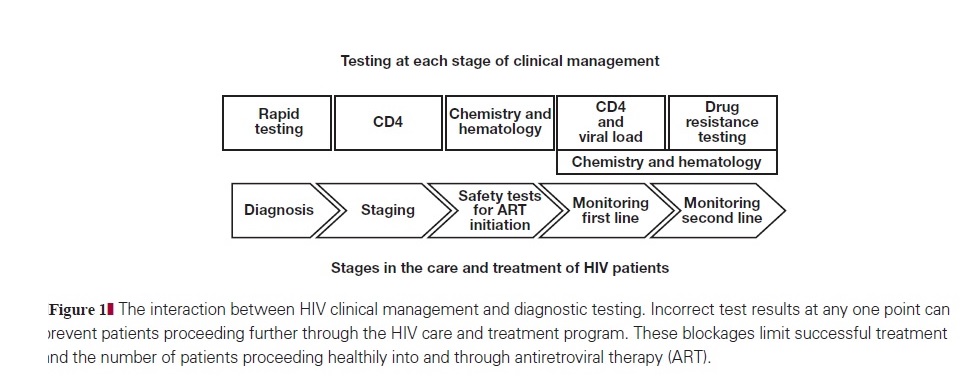
Importance of Reliable Laboratory Testing in Managing HIV Infection for Patient Safety
Reliable laboratory testing is crucial in managing HIV infection at various stages, including diagnosis, disease staging, treatment initiation, and monitoring drug efficacy and toxicity. Poor test performance can lead to reduced treatment effectiveness and denial of appropriate care to patients. Without a reliable diagnosis, patients cannot access most HIV-related services for treatment or prevention, and an accurate CD4 cell count is necessary for correct disease staging and accessing lifesaving antiretroviral drugs. Ongoing laboratory tests provide clinicians with necessary information on drug safety and efficacy during treatment, and the lack of reliable testing may result in poorer treatment outcomes with higher mortality and more frequent illness. Policymakers face the challenge of balancing the benefits of diagnostics against investing in other areas of the health care system, as few studies in resource-limited settings have quantified the usefulness and cost-effectiveness of diagnostics in terms of patient lives or life-years saved.
Impact of Errors in Laboratory Testing on Patient Safety
Errors during laboratory testing can result in serious consequences such as incorrect diagnosis, inappropriate treatment, or the withholding of lifesaving therapy. The preferably analytical and post-analytical stages are where the majority of errors occur, with error rates ranging from 46%-68% and 18%-47%, respectively. Despite years of quality management regulation, errors during the analytical stage still occur, estimated to be between 7% and 12%. In the United States, a small percentage of laboratory errors put patients at risk of inappropriate care and adverse events, while a larger percentage negatively impacts other aspects of patient care.
Why Laboratory Accreditation is important for Improved Test Quality for patient safety?
Governments and organizations working to improve access to essential diagnostics in resource-limited settings should prioritize improving the quality of testing to reduce laboratory errors. Laboratory accreditation is the internationally accepted framework for achieving this. Accreditation can improve patient care by aiding timely and accurate medical decision-making. It can also drive improvements in individual laboratories and have positive effects on other sectors of the healthcare system. Further research is needed to understand the relationship between accreditation and patient care. Ministries of health, donors, and development agencies should prioritize accrediting public laboratories and integrate accreditation programs into policy, planning, and health system strengthening initiatives.
Maintaining Competency of Personnel
It is crucial to ensure that the laboratory personnel maintain their competency to minimize errors and improve the quality of testing. Continuous education and training programs can help personnel stay up-to-date with new technologies, procedures, and regulations. Regular competency assessments can identify knowledge gaps and skill deficiencies and provide opportunities for improvement. Performance feedback, coaching, and mentoring can also help maintain personnel competency. In addition, establishing a culture of quality and safety in the laboratory can motivate personnel to continuously improve their skills and work towards better patient outcomes. Laboratories should prioritize maintaining the competency of their personnel through these various means to ensure accurate and reliable test results.
References
- Agency for Healthcare Research and Quality. 2021 National healthcare quality and disparities report. Rockville (MD).
- Rice J, Lozovatsky M, Hopkins K. Patient portal optimization— Empower patients as partners in health care. Chicago (IL): American Medical Association; 2020.
- DART Trial Team. Routine versus clinically driven laboratory monitoring of HIV antiretroviral therapy in Africa (DART): a randomized non-inferiority trial [published online ahead of print December 9, 2009]. Lancet. 2010; 375:123-131 doi: 10.1016/S0140-6736(09)62067-5.
- Plebani M. Errors in clinical laboratories or errors in laboratory medicine. Clin Chem Lab Med. 2006; 44:750-759.
- Karla J. Medical errors: impact on clinical laboratories and other critical areas. Clin Biochem. 2004; 37:1052-1062.



