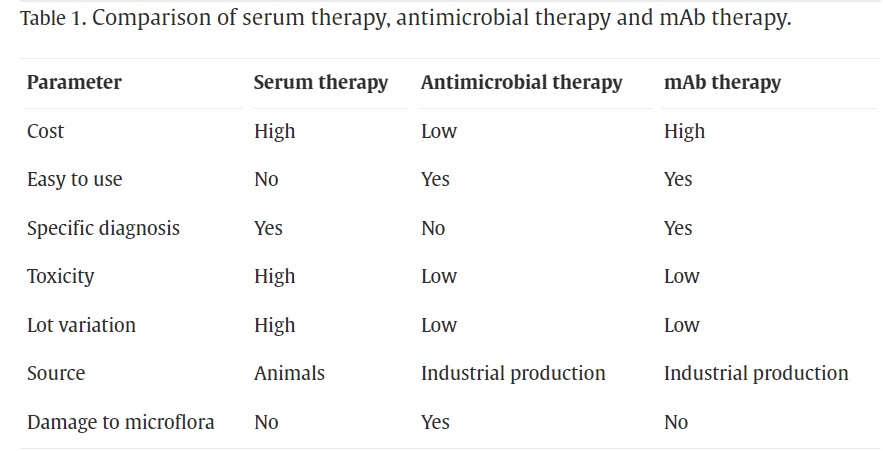
Personalized medicine has the potential to impact the treatment of various diseases and is a popular alternative to standard therapy. Various studies on the pharmacogenomics of monoclonal antibodies (mAbs) and immunoglobulin-containing fusion proteins (igFPs) have been carried out. Monoclonal antibodies (mAbs) as a type of therapeutic protein have revolutionized medicine. Created in the laboratory, they are tailored to adhere to specific targets within the body, such as proteins found on the exterior of cancer cells or viruses. Their specificity renders them very potent in the treatment of various illnesses such as cancer, autoimmune disorders, infectious diseases, and diagnostic tests.
in 1984, the U.S. Food and Drug Administration (FDA) approved the first monoclonal antibody for treating transplant rejection. Since then, more than 80 monoclonal antibodies have been authorized for treating various diseases. Monoclonal antibodies have evolved significantly since their creation through murine hybridoma technology, with improvements in screening techniques and modern engineering resulting in enhanced therapeutic potential of humanized antibodies. While monoclonal antibodies have seen success in treating cancer and immune disorders, efforts to engineer antibodies for fighting pathogens have resulted in four FDA certifications and a growing number of promising clinical trials. Despite advancements, creating anti-infective monoclonal antibodies still presents challenges such as determining the best pathogen targets and conducting more efficient pre-clinical and clinical trials to evaluate the therapeutic advantages.
Advantages of Monoclonal Antibodies Over Traditional Drugs
The potential of monoclonal antibodies (mAbs) as an antibody therapy is vast, particularly in combating microbes that are resistant to antibiotic therapy or emerging viral diseases. In a new era of antimicrobial therapy, mAbs are well-positioned to become crucial agents. Passive serum therapy used to be a popular method for treating microbial infections, but its use decreased with the success of antibiotics and vaccines. However, the development of antibiotic-resistant organisms and the prolonged duration needed for vaccine production have resulted in a renewed interest in antibodies. The rapid discovery of antigens and advancements in engineering the antibody-Fc-domain have created new opportunities to tackle microbial antigen diversity, pathological virulence factors, and transmission, which all contribute to infectious disease morbidity and mortality.
For infectious diseases, monoclonal antibodies have several advantages over traditional drugs. Some are;
- Specificity: MABs can be designed to target specific molecules or antigens on the surface of a pathogen, which reduces the risk of off-target effects on healthy cells. Traditional drugs may target multiple molecules or enzymes, leading to side effects.
- Low toxicity: Monoclonal antibodies are generally less toxic than traditional drugs, as they are metabolized differently and are designed to work specifically in certain areas of the body. This reduces the risk of adverse effects on healthy tissues and organs.
- Reduced risk of drug resistance: MABs are less likely to induce drug resistance in pathogens than traditional drugs, as they target specific molecules that are less likely to mutate.
- Potentially longer-lasting effects: MABs can have a longer half-life in the body than traditional drugs, which means they can provide sustained therapeutic benefits. This reduces the need for frequent dosing and could result in better outcomes for patients.
- Lower risk of drug interactions: Monoclonal antibodies are less likely to interact with other drugs, as they are not metabolized by the same enzymes in the liver as traditional drugs. This reduces the risk of adverse drug interactions.
- Greater potential for combination therapy: Also can be used in combination with other treatments, such as antibiotics or antiviral drugs, to increase their effectiveness. This allows for a more comprehensive approach to treating infectious diseases.
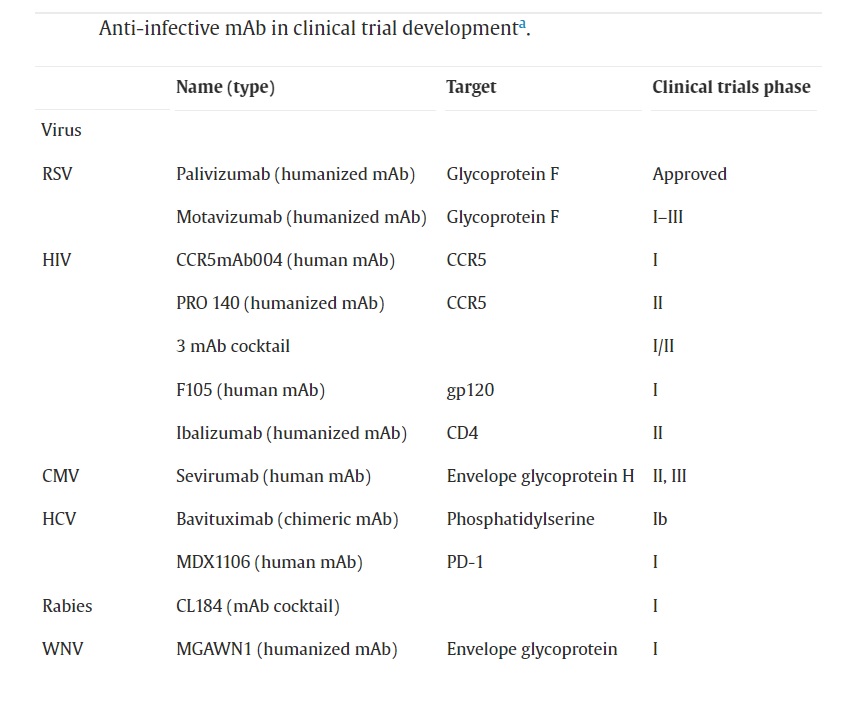
Monoclonal Antibodies for the Treatment of Viral Infections
Monoclonal antibodies show great promise in the treatment of viral infections. The use of mAbs as neutralizing antibodies prevents viruses from attaching to and invading host cells, making it a potentially effective strategy for prophylaxis, or acute treatment of viral illnesses. The development of viral mAbs is currently being pursued in several fields, and there are many ongoing clinical trials.
COVID-19 – The COVID-19 pandemic has caused a global crisis, leading to an urgent need to find ways to prevent and treat infections caused by the SARS-CoV-2 virus. Regulatory agencies such as the FDA and EMA have authorized several monoclonal antibody therapies for emergency use, such as bamlanivimab, etesevimab, casirivimab, and imdevimab, either alone or in combination, which have been shown to reduce the risk of hospitalization and death in certain high-risk COVID-19 patients. Many pharmaceutical companies are currently developing monoclonal antibodies to prevent and treat COVID-19, including IBIO123, a combination of three monoclonal antibodies produced by Immune Biosciences, and Regdanvimab, which targets the spike protein receptor-binding domain of SARS-COV-2 and has been authorized for use in Europe to treat mild to moderate COVID-19. IBIO123 is administered via inhalation and is currently in Phase 1/2 clinical trials for treating outpatients with COVID-19.
Ebola Virus Disease (EVD) – Monoclonal antibodies have been used to treat EVD and also helped improve survival rates and reduce the severity of symptoms in infected individuals. Research conducted on nonhuman primates indicates that the combination of three monoclonal antibodies known as ZMapp could be a potential immune-focused therapy for treating EVD. ZMapp was given to several patients during the 2014-2016 West African Ebola virus outbreak, including some healthcare workers who were infected while treating patients. Also, the mAb cocktail REGN-EB3 was found to be highly effective during the 2018-2019 outbreak in the Democratic Republic of Congo.
Influenza – Human influenza virus causes seasonal epidemics and occasional pandemics, and can infect individuals of any age although children and the elderly are particularly vulnerable. Monoclonal antibodies that target specific strains and subtypes of the influenza virus HA protein can be utilized in the development of new diagnostic tools and in improving the quality control of vaccine production. Multiple panels of mAbs that are specific to influenza HA subtypes and strains have been created for use in evaluating and quantifying the potency of inactivated influenza vaccines.
Respiratory syncytial virus (RSV) – A significant public health concern is the prevention of RSV illness in infants. While palivizumab is a monoclonal antibody that offers RSV prophylaxis, it requires five monthly injections and is only approved for infants with the highest risk of morbidity and mortality from RSV. MEDI8897, on the other hand, is a recombinant human RSV monoclonal antibody with an altered Fc region that prolongs its half-life. It is currently being developed as RSV prophylaxis for all infants.
Human immunodeficiency virus (HIV) – Monoclonal antibody therapy for HIV has been a topic of interest for some time, with several mAbs in development. These mAbs are designed to target various aspects of HIV, such as preventing viral entry, reducing viral load, and possibly even preventing infection in some cases. For example, some viral entry inhibitor mAbs aim to block the cellular receptors CCR5 and CD4 or the viral protein gp120. Ibalizumab was the first monoclonal antibody to be approved for the treatment of HIV-1 infection. In the USA, it is indicated for use in combination with other antiretroviral drugs for individuals with multidrug-resistant HIV-1 infection.
Monoclonal Antibodies for Bacterial Infections
Historically, bacterial diseases mediated by toxins have been successfully treated with specific antibodies and thus remain a promising target for the use of toxin-neutralizing mAbs. Recent studies found that certain mAbs can modify the progression of bacterial and fungal infections, and that immunoglobulins can enhance the effectiveness of other antimicrobial treatments. The question of whether mAbs can work synergistically with traditional antimicrobial drugs is especially relevant since the possibility of developing mAbs as a supplement to current therapies presents an attractive option for improving the outcome of bacterial infections.
Clostridium Difficile Infection(CDI) – Monoclonal antibodies have been used as a treatment for CDI by binding to the toxins created by the bacteria. This binding process prevents the toxins from damaging the intestinal lining and reduces inflammation. CDI is the leading cause of infectious diarrhea in hospitalized patients, and it often recurs after antibiotic therapy. The FDA approved Bezlotoxumab in 2016 as a monoclonal antibody that targets the TcdB toxin produced by Clostridium difficile, for use in conjunction with standard antibiotic therapy to prevent recurrent CDI in adults. Cefotan is a combination of two antibiotics that are effective against a broad range of Gram-negative bacteria and a monoclonal antibody that neutralizes the TcdB toxin generated by Clostridium difficile. It was approved by the FDA in 2020 to treat CDI in adults who have limited or no other treatment options available.
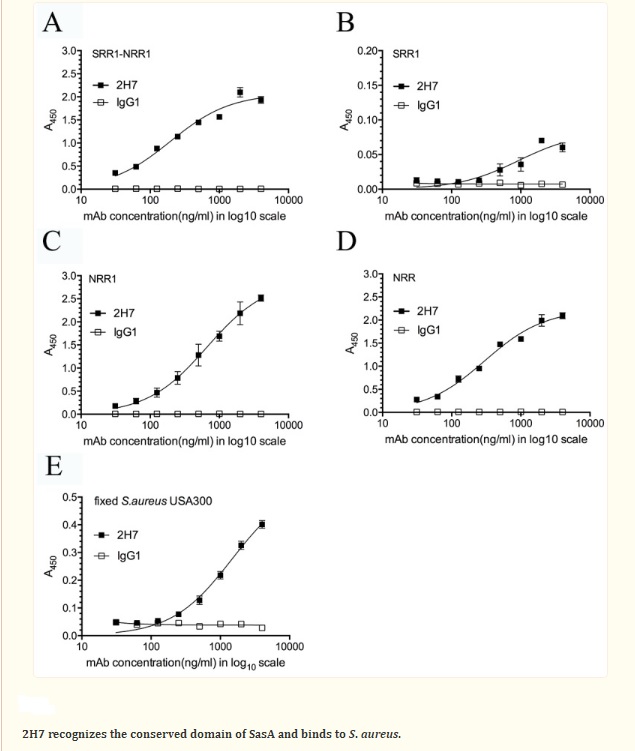
Staphylococcus aureus Infection – Monoclonal antibodies have been created to target the surface proteins of Staphylococcus aureus bacteria, which are responsible for various infections such as skin infections, pneumonia, and sepsis. A potential target for immunotherapeutic approaches against S. aureus infections is Staphylococcus aureus surface protein A (SasA). The study analyzed the sequence of SasA with bioinformatics tools and generated a protective monoclonal antibody (2H7) targeting the conserved domain of SasA. The results suggest that an anti-SasA mAb may be a potential component in an antibody-based immunotherapeutic treatment of MRSA infections. Another monoclonal antibody that has shown potential in early-phase clinical trials as a treatment for S. aureus bloodstream infections is MEDI4893, which targets a surface protein on S. aureus called protein A.
Pseudomonas aeruginosa infection – Pseudomonas aeruginosa is a type of bacteria that can cause a range of infections in humans, including lung infections in people with cystic fibrosis or other chronic lung diseases. One monoclonal antibody currently in development for the treatment of P. aeruginosa infections is called MEDI3902. It targets a specific toxin produced by P. aeruginosa, called Psl, which helps the bacteria form biofilms that are resistant to antibiotics and the immune system.
Challenges in Developing And Using Monoclonal Antibodies For Infectious Diseases
Monoclonal antibodies have shown promise as a potential treatment option for a variety of infectious diseases. However, there are several challenges associated with their development and use. Some of them are as follows;
Identification of appropriate targets: One of the biggest challenges in developing monoclonal antibodies for infectious diseases is identifying appropriate targets. The target should be specific to the pathogen and not present in human cells to avoid off-target effects. Additionally, the target should be conserved across different strains of the pathogen to ensure the monoclonal antibody is effective against all strains.
Difficulty in producing large quantities: Producing large quantities of monoclonal antibodies is another challenge. Monoclonal antibodies are typically produced using mammalian cells, which can be expensive and time-consuming. Additionally, there can be variability in the quality of the antibodies produced, which can affect their effectiveness.
High cost: Monoclonal antibody therapy can be expensive, with some treatments costing tens of thousands of dollars per course of treatment. This cost can limit access to monoclonal antibody therapy, particularly in low- and middle-income countries.
Need for combination therapy: Monoclonal antibody therapy may not be effective as a standalone treatment for infectious diseases. Combination therapy with other treatments, such as antibiotics or antiviral drugs, may be necessary to achieve optimal outcomes.
References
- Motley MP, Banerjee K, Fries BC. Monoclonal antibody-based therapies for bacterial infections. Curr Opin Infect Dis. 2019 Jun;32(3):210-216. doi: 10.1097/QCO.0000000000000539. PMID: 30950853; PMCID: PMC7050834.
- https://www.idsociety.org/covid-19-real-time-learning-network/therapeutics-and-interventions/monoclonal-antibodies/#MonoclonalAntibodiesinDevelopmentforCOVID-19
- https://www.fda.gov/science-research/licensing-and-collaboration-opportunities/anti-influenza-monoclonal-antibodies-hemagglutinin-ha-are-suitable-identification-and-quantification#:~:text=Monoclonal%20antibodies%20(mAbs)%20that%20target,quality%20control%20during%20vaccine%20production.
- Blair HA. Ibalizumab: A Review in Multidrug-Resistant HIV-1 Infection. Drugs. 2020 Feb;80(2):189-196. doi: 10.1007/s40265-020-01258-3. PMID: 31970712.
- https://www.nejm.org/doi/full/10.1056/nejmoa1602615#:~:text=Clostridium%20difficile%20is%20the%20most,toxins%20A%20and%20B%2C%20respectively.
- Yang Y, Qian M, Yi S, Liu S, Li B, Yu R, Guo Q, Zhang X, Yu C, Li J, Xu J, Chen W. Monoclonal Antibody Targeting Staphylococcus aureus Surface Protein A (SasA) Protect Against Staphylococcus aureus Sepsis and Peritonitis in Mice. PLoS One. 2016 Feb 29;11(2):e0149460. doi: 10.1371/journal.pone.0149460. PMID: 26926145; PMCID: PMC4771200.
- Griffin MP, Khan AA, Esser MT, Jensen K, Takas T, Kankam MK, Villafana T, Dubovsky F. Safety, Tolerability, and Pharmacokinetics of MEDI8897, the Respiratory Syncytial Virus Prefusion F-Targeting Monoclonal Antibody with an Extended Half-Life, in Healthy Adults. Antimicrob Agents Chemother. 2017 Feb 23;61(3):e01714-16. doi: 10.1128/AAC.01714-16. PMID: 27956428; PMCID: PMC5328523.
- Phase 1 study of MEDI3902, an investigational anti–Pseudomonas aeruginosa PcrV and Psl bispecific human monoclonal antibody, in healthy adults, Clinical Microbiology and Infection, S.O. Ali, X.Q. Yu, G.J. Robbie, Y. Wu, K. Shoemaker, L. Yu, A. DiGiandomenico, A.E. Keller, C. Anude, M. Hernandez-Illas, T. Bellamy, J. Falloon, F. Dubovsky, H.S. Jafri,



