The Clinical Laboratory Improvement Amendments (CLIA) of 1988 are federal regulatory standards that set quality standards for all laboratory testing performed on humans in the United States, to ensure accurate, reliable, and timely testing results for patient care. CLIA certification is a requirement for all clinical laboratories that conduct testing on human samples, regardless of the laboratory’s size or the number of tests it performs.
CLIA certification is essential for ensuring the quality and safety of clinical laboratory testing. It requires laboratories to meet specific standards for personnel qualifications, facilities, equipment, quality control, quality assurance, and proficiency testing. Compliance with these standards helps to ensure that the laboratory tests are accurate and reliable, which is crucial for making informed clinical decisions about patient care.
CLIA certification also helps to establish a level of trust between patients and healthcare providers. Patients expect that the laboratory tests performed on their samples are accurate and reliable, and CLIA certification helps to ensure that the laboratory meets these expectations.
In addition, CLIA certification is required for laboratories to participate in Medicare and Medicaid programs. This means that laboratories that do not meet the CLIA certification requirements cannot be reimbursed for testing services provided to Medicare and Medicaid beneficiaries. Therefore, CLIA certification is crucial for laboratories to remain financially viable.
What are the different types of CLIA Certification?
Certificates for CLIA compliance are of different types that are available based on the testing performed by the laboratory. A Certificate of Waiver is issued to a laboratory that only conducts waived tests, while a Certificate for Provider-performed Microscopy procedures is issued to a laboratory where a physician, midlevel practitioner, or dentist performs specific microscopy procedures categorized as moderate complexity testing. A Certificate of Registration is issued to laboratories to conduct non-waived moderate and/or high complexity testing until the laboratory is inspected for compliance with CLIA regulations. A Certificate of Compliance is issued to a laboratory that passes a survey by State Agency or CMS surveyors for non-waived testing. A Certificate of Accreditation is issued to a laboratory that performs non-waived moderate and/or high complexity testing and has been accredited by an approved organization by CMS.
Overview of the Regulatory Landscape Surrounding CLIA Certification
The CLIA regulations are federal standards that ensure the accuracy and reliability of laboratory testing. CLIA certification is required for all clinical laboratories that perform testing on human specimens. The certification process involves a survey of the laboratory’s compliance with the CLIA regulations, which can result in penalties or sanctions for noncompliance. Laboratories must also comply with other federal and state regulations, such as HIPAA (The Health Insurance Portability and Accountability Act) and OSHA (Occupational Safety and Health Administration) standards. Compliance with these regulations is critical for maintaining the quality and accuracy of laboratory testing. Failure to comply with CLIA regulations can lead to suspension or revocation of the laboratory’s certification.
Clinical labs that conduct non-waived testing must have proficiency testing (PT) under the CLIA of 1988. This contrasts with CLIA 1967, which required PT only for labs that referred patient specimens across state lines. PT functions as an external check for peer laboratories or reference methods and is an important element in laboratory quality management systems for quality enhancement. PT summary reports enable tracking of long-term analytical performance, and residual PT samples may have additional applications.
CLIA Requirements from Clinical Laboratories
Clinical laboratories must maintain a written procedure manual that outlines the steps for performing all tests, which must be easily accessible and followed by laboratory personnel. The manual must include information on specimen collection, processing, and rejection criteria, as well as procedures for microscopic examinations, test calculations, and result interpretation. It should also cover the preparation of materials used in testing and calibration verification procedures, and control procedures. Additionally, the manual must outline remedial action to be taken if calibration or control results fail to meet the lab’s criteria for acceptability, and include information on limitations in methodologies and reference or normal ranges. The protocol for reporting panic values, as well as criteria for specimen storage and preservation, specimen referral, and reporting of patient results, should also be covered. Finally, the manual should include steps to be taken if a test system becomes inoperable and pertinent literature references.
Step-by-Step Procedure to get CLIA Certificate for Clinical Laboratories
Obtaining CLIA certification involves several steps, including:
Determine the level of certification needed: Laboratories must determine whether they will be performing waived, moderate complexity, or high complexity testing, as this will affect the certification requirements.
Apply for certification: Laboratories must submit an application to the Centers for Medicare and Medicaid Services (CMS) or an accreditation organization approved by CMS, along with applicable fees and supporting documentation.
Prepare for a survey: The laboratory must prepare for an on-site survey (inspection) by CMS or an approved accreditation organization. This includes ensuring compliance with CLIA regulations, having a written procedure manual, and performing proficiency testing.
Undergo survey: During the survey, the laboratory will be evaluated on its compliance with CLIA regulations, including personnel qualifications, facilities, equipment, quality control, quality assurance, and proficiency testing.
Receive certification: If the laboratory passes the survey, it will receive a Certificate of Compliance (COC) or a Certificate of Accreditation (COA), depending on the level of testing performed. Laboratories that perform only waived tests can receive a Certificate of Waiver (COWs).
Maintain certification: Laboratories must maintain compliance with CLIA regulations to keep their certification. This includes regular proficiency testing, reporting adverse events, and undergoing periodic surveys.
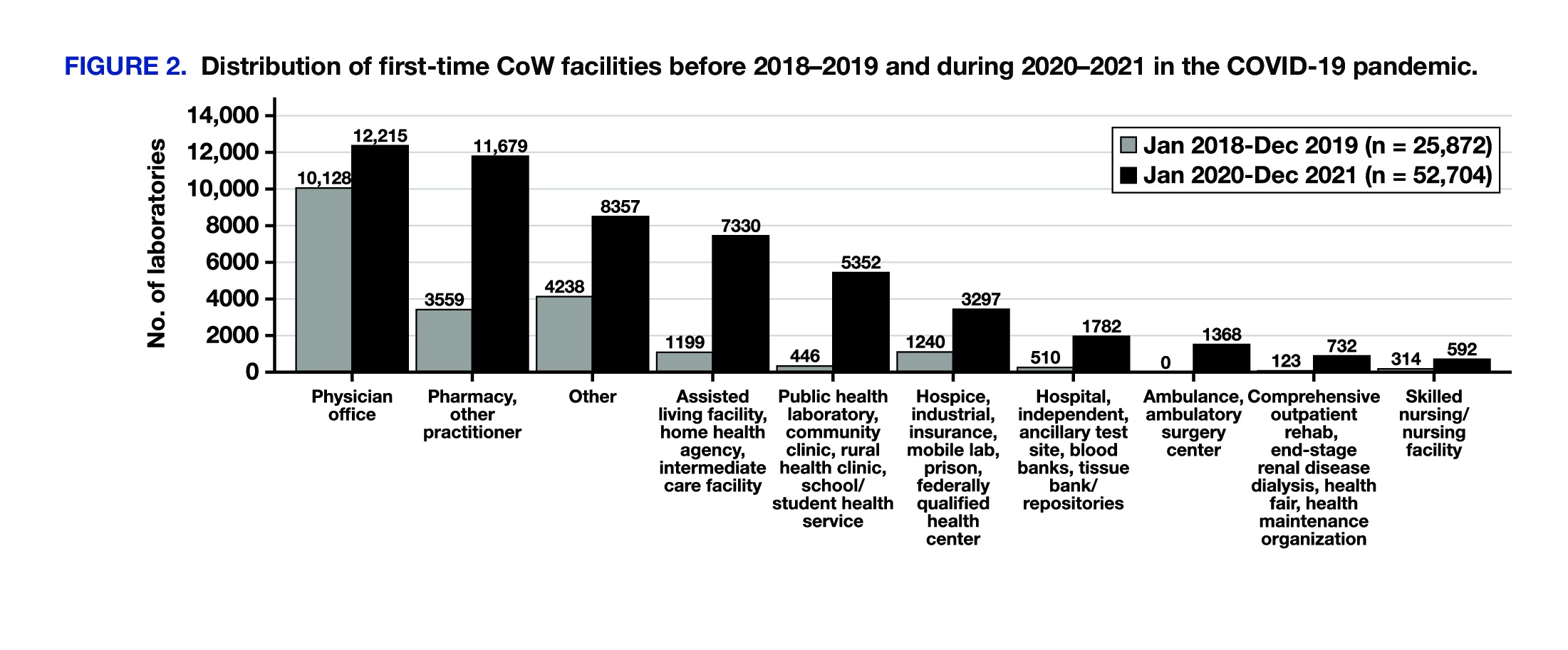
Increase in Certificate of Waiver (CoWs) reports
It’s important to note that the specific requirements and process for obtaining CLIA certification may vary depending on the type of laboratory and the state in which it is located. The number of first-time CLIA Certificates of Waiver (CoWs) increased by 73% from 2019 to 2020 and by 39% from 2020 to 2021. The total number of first-time CoWs during 2020-2021 was more than twice what it had been in 2018-2019. All facility categories experienced a dramatic rise in first-time CoWs in 2020-2021 compared with 2018-2019, with the highest increase observed in assisted living facilities/home health agencies/intermediate care facilities. The total test volume reported by all first-time CoWs increased from 29 million in 2019 to 204 million in 2021, with a 118% increase from 2020 to 2021.

Ensuring Compliance with CLIA Regulations in Clinical Laboratories
To ensure ongoing compliance with CLIA regulations, it is important to stay up-to-date with any changes and conduct regular staff training, develop and implement written policies and procedures, perform regular internal audits, participate in proficiency testing programs, implement corrective actions, establish a culture of quality and continuous improvement, maintain proper documentation, have a plan in place to address deficiencies, and engage with accrediting organizations and regulatory agencies. By following these protocols, clinical laboratories can maintain compliance with CLIA regulations and provide high-quality testing services to patients.
Failure to comply with the minimum standards set by CLIA can result in severe consequences for clinical laboratories, including fines, institutional sanctions, and suspension of testing privileges. All medical laboratories, including POCT facilities, are subject to unannounced inspections by authorized organizations, such as the CMS, the Joint Commission, or CAP, as mandated by CLIA. These organizations are approved by CMS and have standards that meet or exceed those of CLIA. Therefore, compliance with these standards is essential for clinical laboratories to ensure patient safety and maintain the ability to conduct testing.
References
- Clinical Laboratory Improvement Amendments of 1988. Pub. L. No 100–578, 102 Stat.2903 (October 31, 1988).
- Centers for Medicare and Medicaid. Clinical laboratory improvement amendments (CLIA). https://www.cms.gov/Regulations-and-Guidance/Legislation/CLIA/index.html (Accessed March 2016).
- Boone DJ. Literature review of research related to the Clinical Laboratory Improvement Amendments of 1988. Arch Pathol Lab Med 1992; 116: 681–93.
- Clinical and Laboratory Standards Institute. Using proficiency testing to improve the clinical laboratory: approved guideline: second edition CLSI document GP27-A2 Wayne, PA: Clinical and Laboratory Standards Institute; 2007. p. 1–45.
- Yang Xia, Ph.D., Nancy Anderson, MMSc, Increases in CLIA-Waived Testing Sites Since the Start of the COVID-19 Pandemic, Laboratory Medicine, Volume 54, Issue 2, March 2023, Pages 126–129, https://doi.org/10.1093/labmed/lmac154.
- Ehrmeyer SS, Laessig RH. Regulatory requirements (CLIA ’88, JACHO, CAP) for decentralized testing. Am J Clin Pathol 1995; 104(Suppl): S40-S49.
- Joint Commission on Accreditation of Healthcare Organizations. Comprehensive accreditation manual for pathology and clinical laboratory services. Oakbrook Terrace: JACHO,2001.
- Joesph Wiencek & James Nichols (2016): Issues in the practical implementation of POCT: overcoming challenges, Expert Review of Molecular Diagnostics, DOI:10.1586/14737159.2016.1141678.



