Polymerase Chain Reaction (PCR) has become an important tool for targeting and analyzing specific genes and gene sequences. Optimal PCR results are achieved by optimizing the reaction conditions which can influence the specificity and efficiency of this reaction.
The process of PCR optimization includes determining the appropriate concentration of primers, annealing temperature, MgCl₂ concentration, template concentrations, and polymerase among several other factors. If PCR optimization is not performed correctly, it can lead to various problems, such as the absence of PCR products or inefficient amplification of the target template, the presence of nonspecific bands or fuzzy background, the creation of “primer-dimers” that compete with the intended target, or mutations due to incorrect nucleotide incorporation.
Optimization of each PCR reaction is crucial and necessary, and it should be tailored according to the chosen template and primer pairs. The optimization process is especially significant for repetitive diagnostic or analytical procedures where precise and efficient amplification is critical.
Goals of PCR Optimization
The goals of PCR optimization are to maximize specificity and efficiency, such that the amplified product is a replica of the intended target sequence. However, this task is not always easy, as numerous factors have a potential impact on the success of the reaction. Achieving maximally specific and efficient amplification while minimizing non-specific products can be thought of as “balancing a chain of dominos”. If one parameter in the chain is changed or out of balance, it reflects further changes down the chain which then leads to undesired effects during or after amplification.
In practice, optimizing different types of PCRs can involve anything from decreasing reaction temperatures slightly below the ideal annealing temperature for primer hybridization to titrating various concentrations of Mg and other ions for enhanced activity of DNA polymerase. It is important to understand how each factor affects the PCR product so that corrective measures can be taken when needed. Furthermore, it is also beneficial to explore different sets of cycling conditions and compare overall reaction performance to choose the best settings for achieving effective and reproducible results.
Factors Affecting PCR Specificity and Efficiency
The process of optimizing a specific PCR can be a challenging and time-consuming task because it involves numerous parameters. These parameters include factors such as;
- The quality and concentration of the DNA template,
- The design and concentration of primers
- The concentration of magnesium ions
- The concentration of the four deoxynucleotides
- The type of PCR buffer systems used
- The selection and concentration of DNA Polymerase
- The PCR thermal cycling conditions and
- The use of the “hot start” technique

Template DNA Quality and Length
When it comes to PCR optimization for accuracy, template DNA quality and length of the DNA play an important role since they influence how well the primers bind to their targets. Poor quality of template DNA can lead to nonspecific or low-efficiency amplification, resulting in a false negative or false positive respectively. Furthermore, if the genes of interest are too short, then these short sequences will not be efficiently amplified, leading to a false negative.
Accordingly, it is important to ensure that you are working with good-quality extracted DNA and your gene should also meet the recommended length of 200bp to 500bp for efficient amplification. In scenarios where the target sequence is shorter than 200bp, proofreading enzymes may need to be included in the PCR reaction to increase specificity. Inversely, if the PCR fragment is larger than 150-500 bp, then more time and higher temperatures are needed for both strands at each end of the fragment to be dissociated simultaneously, leading to lower product yields.
Primer Design and Concentrations
When it comes to PCR optimization, primer design, and concentrations are key for achieving accuracy in results. Primer design involves selecting complementary primers with the correct sequence that efficiently anneal to your template DNA without producing spurious products.
The ability of an oligonucleotide to bind to a DNA template and initiate polymerization depends on two factors: the speed at which the primer separates from the primer-template complex before polymerization begins, and the rate at which the DNA polymerase elongates the primer to form a stable primer-template complex. The process of PCR is controlled by these kinetics, where the initial binding of primer and template DNA is temporary, but the addition of the first few nucleotides creates a strong and stable complex, allowing the primer to be extended until the final product is formed on the template.
Regarding primer concentrations, there is some debate as to what concentration of primers should be used. On one hand, too much primer decreases product specificity while insufficient amounts result in lower yields.
However, a recent study found that concentrations between 0.2 and 1uM led to increased PCR efficiency, suggesting that low primer concentrations can reduce non-specific product formation in a PCR reaction. But both cases have varying degrees of success so it’s important to optimize each based on the target amplicon length, desired sensitivity, and gene copy number.
Reaction Conditions & Temperatures
For most DNA fragments that range from 100-500 by, the optimal PCR temperature for annealing is usually between 55°C – 65°C with an optimal elongation temperature of 72°C. Generally, lower temperatures are more specific for certain targets, but may result in lower yield; whereas higher temperatures can provide increased yield at the expense of a reduction in specificity. For amplifications longer than 1kb, a standard annealing temperature can range from 55°C to 58°C with elongation at 72°C. If one or two primers are known to produce high specificity results, then a higher annealing temperature (59 – 62°C) should be used for these specific primers while keeping the remaining primers at optimum targeted product size and yield.
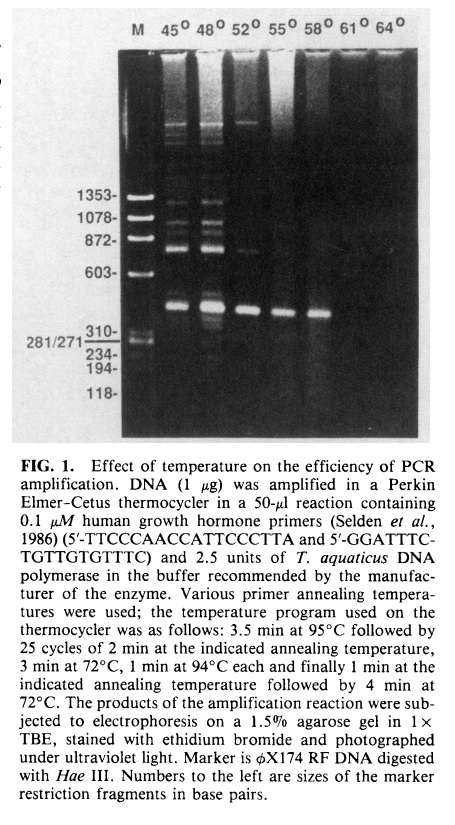
The magnesium chloride concentration also greatly impacts the outcome of the PCR reaction and plays a role in maintaining consistency between reactions by controlling nucleic acid hybridization affinity and thereby affecting the polymerase activity itself. The recommended starting point for magnesium concentration is 2mM although concentrations as low as 0.5mM and as high as 5mM may be used depending on primer composition.
Measures to Improve PCR Specificity or Efficiency
Improving the specificity and efficiency of polymerase chain reaction (PCR) result is a key step in achieving accurate results. With optimized reaction conditions, different measures can be taken to improve the PCR process.
One strategy to improve PCR specificity includes manipulating PCR components to reduce non-specific bindings. This can involve altering components such as concentrations of magnesium ions, dNTPs, buffer pH, salts, detergents, and primer design among others. Ions such as Mg2+ in particular play a major role in stabilizing duplexes formed during hybridization and modulating base stacking during strand separation. Thus, alterations to Mg2+ levels or other components become especially critical when optimizing PCR protocols to achieve maximum efficiency and eliminate non-specific bindings.
Also, strategies may be used to enhance signal strength and reduce background noise for improved amplification during Cycling. During this process, there might also be a tendency for mispriming events which lead to nonspecific amplification.
Aliquoting Alternatives & Buffering Solutions
When aiming to optimize the PCR process for accurate results, aliquoting and buffering solutions can be an effective solution – although the two tactics may be better suited to certain applications than others. Aliquoting involves preparing several multiple test tubes with identical reagent mixtures beforehand, while buffering solutions are substances that stabilize a pH level in reactions by controlling the amount of acid or base added.
For certain applications, aliquoting can provide trackable samples with complete accuracy and precision, while also avoiding contamination risks between testing samples. However, applying aliquoting is a laborious task that can take up time and resources that might better be used elsewhere in some cases.
Similarly, buffering solutions have their practical merit, as they can control conditions such that they are more precise and predictable throughout the entirety of the reaction period. This predictability increases confidence in results as it eliminates potential errors due to unexpected pH levels. But buffering solutions also come at a cost: there is always a risk of taking away from the desired result should an insufficiently-tested buffer substance increase sample amounts by too high a degree.
Changing Reaction Time or Amounts
In terms of reaction time, research has suggested that increasing the extension time in a PCR experiment can help amplify longer and more specific DNA sequences while maintaining efficiency. However, extending the reaction time too much also increases the possibility of non-specific amplifications which will decrease accuracy. It is important to find the time between minimal but effective and overamplified when considering changing the reaction time.
Whether trying to increase reaction time or quantities of reagents added to a PCR reaction, it is important to strike a balance between providing ample materials for accurate results and not flooding the system with too many unnecessary materials that may cause inaccuracies. As an additional factor to consider when researching and performing PCR experiments, other considerations such as proper instrument calibration must also be addressed in efforts of achieving accurate results.
Conclusion
A research study discovered that mono- and disaccharides can be useful as enhancers for PCR in most cases. Unlike oligo- and polysaccharides, low molecular weight carbohydrates can speed up amplification and boost the amount of products obtained. The carbohydrates’ ability to enhance PCR is not dependent on their ability to reduce. Sucrose was found to be the most effective carbohydrate for producing specific and dependable amplification. As the size of the amplification area increases, the impact of carbohydrates becomes less significant.
The best PCR outcome is achieved when it has high specificity, yield, and fidelity. The target sequence’s nature and each component of PCR can affect its specificity, yield, and fidelity. Sometimes, the conditions that maximize yield cannot be used to achieve high fidelity or specificity, and optimizing conditions for fidelity can decrease efficiency. Therefore, it’s crucial to plan to achieve the required specificity, yield, and fidelity for the intended PCR application.
Choosing the appropriate polymerase for PCR depends on understanding the PCR’s purpose. Unfortunately, T4 polymerase, the most precise enzyme studied to date, and T7, the most efficient polymerase, are not thermostable. Among the remaining thermostable polymerases, Vent or Pfu would be the preferred enzyme if fidelity is a concern, while Taq would be adequate if the PCR’s purpose is to generate a large amount of a specific target sequence.
References
- Grunenwald, H. (n.d.). Optimization of Polymerase Chain Reactions. PCR Protocols, 89–100. doi:10.1385/1-59259-384-4:89
- Sakhabutdinova, A. R., Chemeris, A. V., &Garafutdinov, R. R. (2020). Enhancement of PCR efficiency using mono- and disaccharides. Analytical Biochemistry, 113858. doi:10.1016/j.ab.2020.113858
- WU, D. Y., UGOZZOLI, L., PAL, B. K., QIAN, J., & WALLACE, R. B. (1991). The Effect of Temperature and Oligonucleotide Primer Length on the Specificity and Efficiency of Amplification by the Polymerase Chain Reaction. DNA and Cell Biology, 10(3), 233–238.doi:10.1089/dna.1991.10.233
- Specificity, Efficiency, and Fidelity of PCR Rita S. Cha and William G. Thilly Center for Environmental Health Sciences and Division of Toxicology, Whitaker College of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139