Precision medicine (PM) is a novel strategy for preventing and treating diseases. It seeks to categorize pharmacological therapies into specific subgroups of patients who have the relevant genetic variants, thus moving beyond the conventional “one size fits all” drug model. This system employs patient-specific data to create customized treatments, and antibody-based drugs are now an essential part of precision medicine in cancer care.
PM can be considered the ultimate field of science, with potential benefits that go beyond targeting therapies for patients, including the ability to detect healthy individuals at high risk and take preventive measures. The fundamental concept of PM is that a person’s genetic makeup primarily affects the occurrence of diseases. Recent strides in characterizing individual variations in genomic sequences should broaden the scope of PM from rare monogenic ailments to more prevalent and genetically complicated pathologies.
In the past, cancer patients were usually treated based on their tumor’s origin and histological subtype. Nevertheless, rapid advances in molecular technology have resulted in clinical-grade tests that can examine the immune profile and identify its specific alterations. Furthermore, conventional oncology practices often are based on decisions that benefit the average population, frequently obtained from randomized clinical trials of unselected patients, which have been the cornerstone of drug approvals for many years.
Cancer treatment has been the most extensively studied area of PM because tumors typically result from genetic variations. Biologic therapies, such as antibody-based drugs, can target specific proteins in cancer cells by either inhibiting their activity or eliciting an immune response. Compared to conventional chemotherapy, this targeted approach can reduce side effects and limit harm to healthy cells.
For example, the drug trastuzumab targeting the HER2 protein in breast cancer cells has significantly improved outcomes for patients with HER2-positive breast cancer. Another antibody-based drug, rituximab, targets the CD20 protein in B cells and has revolutionized the treatment of non-Hodgkin’s lymphoma. More recently, a new generation of antibody-based drugs known as bispecific antibodies has emerged which can target multiple proteins at once. This shows promising results for treating a variety of cancers, including acute lymphoblastic leukemia, multiple myeloma, and solid tumors.
Monoclonal Antibodies: A Revolutionary Approach to Targeted Cancer Therapy
The developments in targeted cancer therapy have undergone a revolutionary transformation over the last 30 years. The therapeutic application of monoclonal antibodies (mAbs) became possible after the introduction of the hybridoma technique by Kohler and Milstein in 1975. Greg Winter’s antibody humanization technique, which debuted in 1988, was another significant advancement in the development of therapeutic mAbs for various types of cancer. Monoclonal antibodies are approved in large numbers and are used to treat many types of tumors and chronic illnesses. The pharmaceutical industry anticipates that biological markers will play a significant role in optimizing the use of mAbs in clinical practice.
The FDA has already sanctioned more than a dozen mAbs for treating both hematological and solid malignancies, and numerous new mAbs are being studied in clinical trials. Monoclonal antibody and gene transfer technologies have further facilitated the exploration of fundamental knowledge on antigen recognition, T cell activation, and T cell co-stimulation, resulting in the success of checkpoint blockade and CAR T cell therapy. There are various types of mAbs used in cancer treatment, such as naked, conjugated, and bi-specific mAbs. Naked mAbs are the most commonly used mAbs to treat cancer, and they work by enhancing the immune response against cancer cells. Alemtuzumab is a naked mAb that binds to the CD52 antigen on lymphocytes and is used to treat chronic lymphocytic leukemia (CLL). Other naked mAbs work primarily by binding to and obstructing antigens on tumor cells, such as trastuzumab, an antibody that targets the HER2 on breast and stomach cancer cells. Conjugated mAbs are those that bind to a chemotherapy agent or a radioactive particle and transport one of these substances straight to the cancer cells. Chemolabeled antibodies are those antibodies with potent chemotherapy drugs attached to them, such as brentuximab vedotin, an antibody that targets the CD30 antigen found on lymphocytes.
The Advancement of Antibody-Based Cancer Therapies
Over half of all immunoglobulins in human blood are made up of the IgG1 molecule, which is the most common format for both natural and synthetic antibodies. Among the 43 FDA-approved antibody-based cancer therapies, 30 use the IgG1 format. In the last twenty years, progress in the engineering and production of antibodies has enabled the accurate identification and mass production of post-translational modifications (PTMs). This has resulted in the widespread application of antibodies for cancer treatment and other areas of medicine. While natural antibodies typically activate the innate immune system, the discovery of antibodies that activate components of the adaptive immune system, especially cytotoxic T cells, is a significant breakthrough in cancer therapy. The use of antibody-based cancer therapies has been steadily increasing, and next-generation antibody engineering and personalized combination treatments for individual cancer patients are expected to drive the success of these drugs.
Current State and Future Directions of Antibody-Based Therapies in Cancer Treatment
Antibody-based therapies have shown great promise in cancer treatment, but their full potential is yet to be explored. Several therapeutic antibodies are being tested in clinical trials, both in the early and late stages. These antibodies generally have different and milder toxicities than traditional chemotherapeutic agents. Regulatory bodies such as the FDA typically require large Phase III trials demonstrating an overall survival benefit compared to standard therapy for therapeutic antibodies to be approved for use. Antibodies targeting the ERBB family (including EGFR) and VEGF have been most successful in treating solid tumors. For instance, studies have shown that patients with colorectal cancer treated with EGFR-specific antibodies who have wild-type KRAS exhibit improved responses. Therefore, the uses of these agents are limited to individuals with colorectal cancer where KRAS is not mutated. Similarly, trastuzumab’s use has been restricted to those with high levels of ERBB2 expression. Based on the clinical success of these antibodies and preclinical data demonstrating the improved tumor response of combined signaling blockade with antibodies to different receptors, various clinical trials of antibodies as combination therapies are currently underway.
Also, there are antibodies approved for cancer indications in countries other than the United States. For instance, in the European Union, Catumaxomab, is approved for the treatment of malignant ascites caused by an EPCAM-positive tumor. Additionally, Nimotuzumab, a humanized IgG antibody that targets EGFR, is approved for the treatment of head and neck cancer, glioma, and nasopharyngeal cancer in several countries.
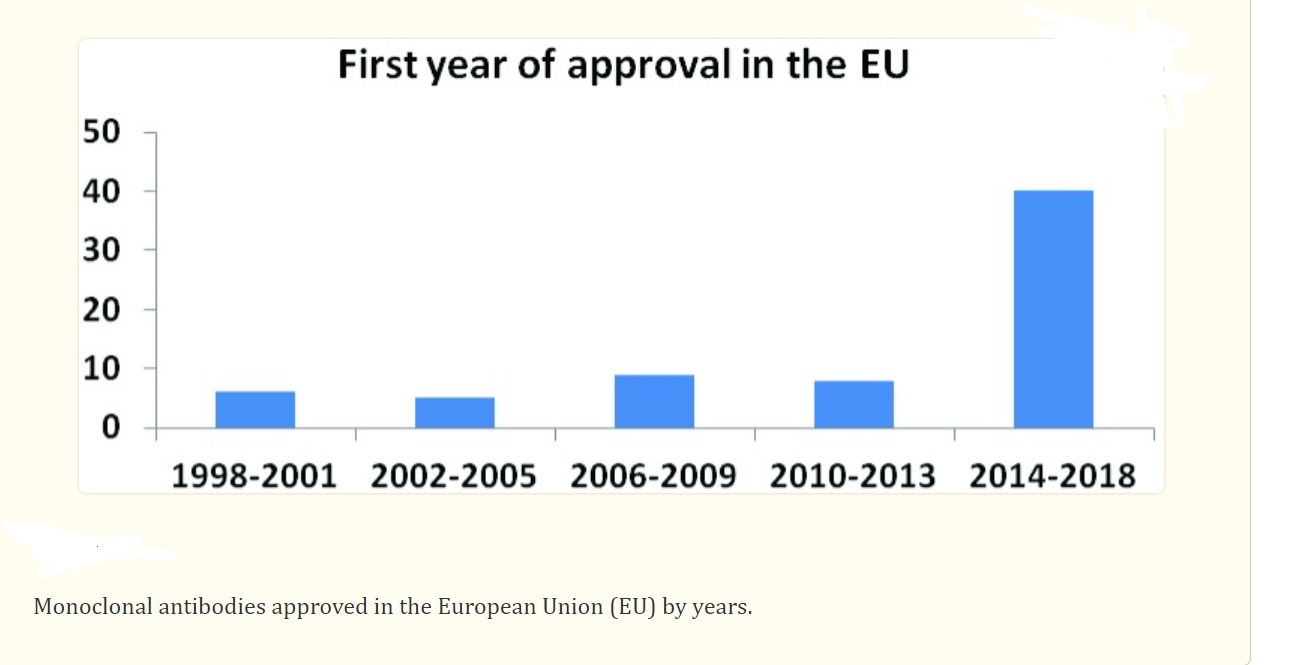
Advantages of Antibody-Based Drugs Over Traditional Chemotherapy
Although traditional chemotherapy is widely used for cancer treatment, it has several limitations, including lacking specificity and destroying the healthy cells. In contrast, antibody-based drugs offer several advantages over traditional chemotherapy. Some of them are as follows;
Targeted therapy: Antibody-based drugs specifically target cancer cells thus minimizing the damage to normal cells and avoiding side effects such as nausea, vomiting, and hair loss.
Increased efficacy: Antibody-based drugs are highly effective by targeting the cancer cells’ specific proteins, signaling pathways, and/or checkpoints, disrupting the cancer cells’ growth and survival.
Reduced drug resistance: Resistance to chemotherapy can develop due to the toxic effect on healthy cells and the heterogeneity of cancer cells. Antibody-based drugs are less likely to induce drug resistance because they target specific molecules responsible for the growth and survival of cancer cells.
Improved quality of life: Due to the reduced side effects of antibody-based drugs, patients can have a better quality of life during and after treatment. They may be able to continue their everyday activities with fewer disruptions to their routine.
Challenges in Precision Medicine and Cancer Therapy
One major challenge is the lack of access to advanced genomic testing and data analysis for all patients. While genomic testing is becoming increasingly available and affordable, it is still not universally accessible. Moreover, individuals living in remote regions may not have equal opportunities to receive the same level of testing and analysis as those residing in urban or academic medical facilities. This can create disparities in healthcare provision and treatment outcomes.
Furthermore, the interpretation of genomic data can be complex requiring specialized expertise, which may not be available at all healthcare facilities. The development of more user-friendly and accessible tools for genomic data analysis will be critical for the broader implementation of precision medicine.
Another challenge is the need to develop new therapies that target the specific genetic and molecular alterations driving each patient’s tumor. However, many genetic alterations have yet to be effectively targeted. This requires ongoing research and innovation in drug development and personalized medicine.
Furthermore, precision medicine may not be effective for all patients, as tumors can develop resistance to targeted therapies over time. There is a need for ongoing monitoring and adaptation of treatment plans to account for changes in tumor biology and drug resistance.
In addition to these scientific challenges, there are also ethical and regulatory considerations. For example, there is a need to ensure the privacy and security of patient data, as well as to address concerns about the potential for discrimination based on genetic information.
Cost Considerations of Antibody-based Drugs: Antibody-based drugs are typically more expensive than traditional small molecule drugs, in part because they are more complex to manufacture. They also require larger doses and longer treatment durations. Additionally, some antibody-based drugs are only approved for use in certain patient populations, which can limit their market and drive up their cost. For example, a drug that is only approved for use in a small subset of patients with a particular type of cancer may have a higher cost per patient than a drug that is approved for use in a larger patient population.
Current Research and Future of Precision Medicine in Cancer Therapy
Our limited knowledge of how cancer genes and traits are related prevents us from effectively using genetic information to match cancer patients with the most suitable treatments. To overcome this drawback, it is possible to subject living tumor cells from patients to potential treatments to test their effectiveness. Recently, advanced functional diagnostic technologies have emerged, which include innovative ways to manipulate tumors, and precise assays to evaluate tumor responses at a molecular level that surpass the limitations of older chemosensitivity tests. These technologies offer significant potential for combining functional testing with next-generation sequencing and immunoprofiling to accurately match combination therapies with individual cancer patients in the future.
One of the promising areas of research is the development of liquid biopsies, which involve testing a patient’s blood for cancer biomarkers instead of taking a tissue sample. This approach is less invasive and more convenient than traditional biopsies, and allows for more frequent monitoring of the cancer’s progression and response to treatment.
Artificial intelligence and machine learning are also being used in the field of precision medicine to analyze large amounts of data, including genomic data, medical records, and clinical trial data. This allows physicians to identify patterns and predict treatment outcomes, which can help them make more informed treatment decisions.
New methods for inhibiting immune checkpoint regulators, utilizing engineered T-cell therapy to overcome immune tolerance, and discovering fresh tumor antigens through next-generation sequencing have ushered in a new phase of cancer immunotherapy. This area includes both passive and active immunotherapy.
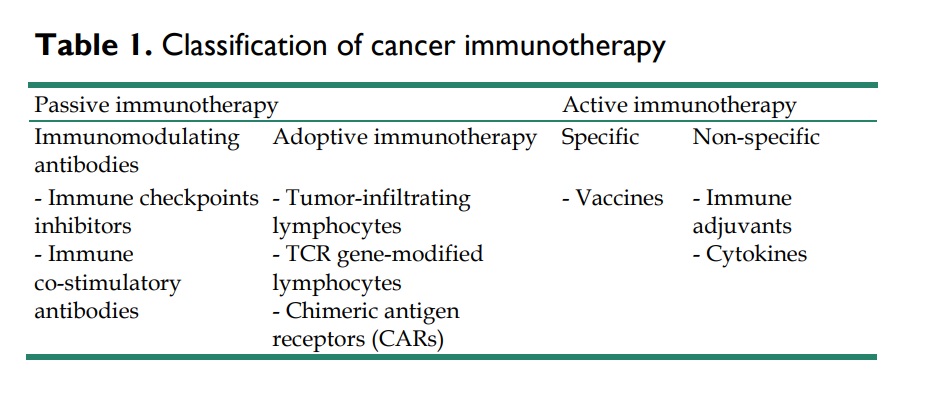
Passive immunotherapy involves introducing agents like monoclonal antibodies, lymphocytes, or cytokines that strengthen the existing anti-tumor response. Conversely, active immunotherapy strives to activate the individual’s immune system to fight tumor cells using methods like vaccination, non-specific immunomodulation, or targeting specific antigen receptors.
As our understanding of cancer biology and genetics continues to advance, we can expect to see more personalized and effective treatments for individual patients. While challenges remain, the potential benefits of precision medicine in cancer make it an exciting and important area of research.
References
- Friedman, Adam A.; Letai, Anthony; Fisher, David E.; Flaherty, Keith T. (2015). Precision medicine for cancer with next-generation functional diagnostics. Nature Reviews Cancer, (), –. doi:10.1038/nrc4015
- Jin, S., Sun, Y., Liang, X. et al.Emerging new therapeutic antibody derivatives for cancer treatment. Sig Transduct Target Ther 7, 39 (2022). https://doi.org/10.1038/s41392-021-00868-x
- Fountzilas, E., Tsimberidou, A.M., Vo, H.H. et al.Clinical trial design in the era of precision medicine. Genome Med 14, 101 (2022). https://doi.org/10.1186/s13073-022-01102-1
- Rebecca S. Goydel;Christoph Rader; (2021). Antibody-based cancer therapy . Oncogene, (), –. doi:10.1038/s41388-021-01811-8
- Zhang H, Chen J. Current status and future directions of cancer immunotherapy. J Cancer. 2018 Apr 19;9(10):1773-1781. doi: 10.7150/jca.24577. PMID: 29805703; PMCID: PMC5968765.



