The idea of “Precision Oncology” or the application of “Precision Medicine” to cancer treatment has gained popularity due to its potential to identify the specific oncogenic mutation in a patient’s cancer genome and target it with selective drugs. However, critics warn that this approach oversimplifies the complexity of cancer and the heterogeneity of tumors, as well as the development of drug resistance. There are emerging concepts of tumor dynamics that challenge the conventional paradigm of oncogene-driven tumorigenesis, such as intratumor genetic heterogeneity and phenotypic plasticity of cells. Cancer is not just a disease of DNA or cells but of the tissue itself. The failure to eradicate a tumor may be due to the robust and adaptive ecosystem of diverse, communicating cells that can sense and repair themselves, which could lead to recurrence even after attempts to kill cancer cells.
Overview of Antibody-Drug Conjugates (ADCs)
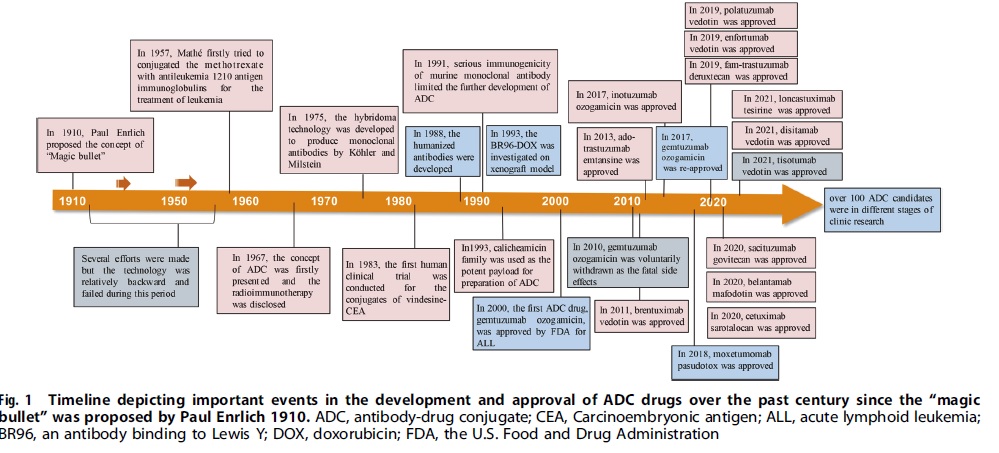
The approval of Mylotarg (gemtuzumab ozogamicin) by the FDA in 2000 marked the beginning of the ADC era of cancer-targeted therapy. Since then, there have been 14 ADC drug approvals for both hematological malignancies and solid tumors worldwide, with over 100 ADC candidates in different stages of clinical trials. ADC is leading a new era of targeted cancer therapy and is expected to replace conventional chemotherapies in the future.
A desirable ADC drug should be stable in the blood, accurately reach the intended therapeutic area, and discharge its cytotoxic substances close to the target (for instance, cancer cells). Each element can affect the efficacy and safety of the ADC, so the development process must consider all key components including choosing the target antigen, antibody, cytotoxic payload, linker, and conjugation methods.
Targeted Therapy for Solid Tumors: The Advantages of ADCs over Conventional Chemotherapy
For the last 50 years, chemotherapy using cytotoxic compounds has been the primary method for systemic cancer treatment, with combinations of different agents developed to maximize antitumor activity. However, these treatments are only partially effective in solid tumors due to limited doses and systemic toxicity, resulting in few long-term remissions in metastatic disease patients. Medicinal chemists and oncologists have sought ways to increase cytotoxic delivery to cancer cells while minimizing exposure to healthy tissue. Monoclonal antibodies presented an opportunity to exploit their specific binding properties for selective delivery of a cytotoxic agent to cancer cells through chemical conjugation, creating an antibody-drug conjugate (ADC).
How Antibody-Drug Conjugates Work?
ADCs have a specific targeting role and efficient killing effect on cancer cells, similar to a precision-guided biological missile that can accurately destroy cancer cells while reducing off-target side effects. The ADC is composed of an antibody, cytotoxic payload, and chemical linker, all of which affect the final efficacy and safety of the drug. Once the ADC’s mAb binds to specific antigens expressed on cancer cells, it is internalized by cells and undergoes endocytosis, maturing into late endosomes and fusing with lysosomes. The cytotoxic payloads are released via chemical or enzyme-mediated processes, inducing cell apoptosis or death by targeting DNA or microtubules. Additionally, the Fab segment of the antibody can bind to antigen epitopes of virus-infected or tumor cells, while the Fc segment binds to Fc receptors on killer cells, mediating direct killing effects. The antibody component can also inhibit downstream signal transduction of antigen receptors, such as PI3K or MAPK, to induce cell apoptosis.
Structure and Key Components of ADCs
An antibody-drug conjugate (ADC) consists of three main components: an antibody, a cytotoxic payload, and a chemical linker. To be effective, an ADC must remain stable in circulation, accurately target the therapeutic site, and release the cytotoxic payload close to the target (such as cancer cells). The efficacy and safety of the ADC can be influenced by each of these components, and all of them must be carefully considered during development. This includes the selection of the target antigen, antibody and cytotoxic payload, linker, and conjugation methods.
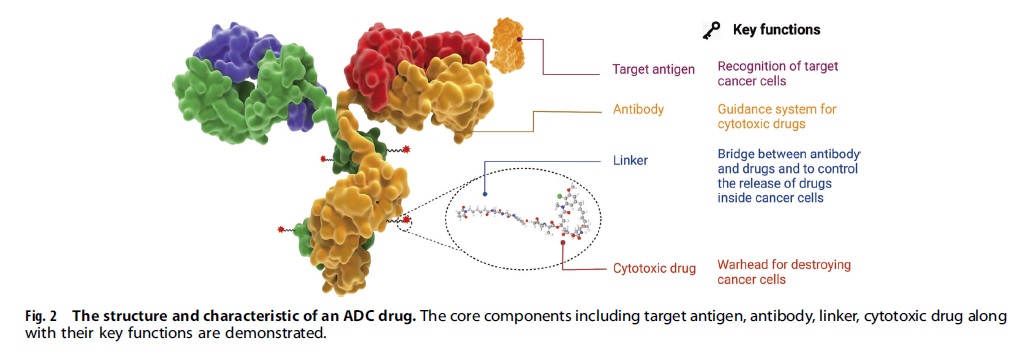
Selecting an appropriate target antigen is crucial for ADC design, as it determines the delivery mechanism of cytotoxic payloads. The ideal target antigen should be highly expressed in tumor cells, non-secreted, and internalized upon binding. Approved ADC drugs typically target overexpressed proteins, but emerging evidence suggests targeting the tumor microenvironment may also be valuable. Targeting these tissues could broaden efficacy and reduce drug resistance.
The antibody moiety in ADCs should have high binding affinity, facilitate efficient internalization, be non-immunogenic, and have a long plasma half-life. Early ADCs used mouse-derived antibodies, but now chimeric and humanized antibodies are preferred for reduced immunogenicity. The IgG1 subtype is commonly used due to its strong effector functions. High antigen affinity may reduce tumor penetration, and miniaturized antibodies can improve penetration but may also reduce half-life. Therefore, various factors should be considered when designing ADCs with miniaturized antibodies.
Linkers are crucial for connecting the antibody and cytotoxic drug in ADCs and a stable and effective linker is essential for their success. There are two types of linkers: cleavable and non-cleavable. Cleavable linkers take advantage of differences between systemic circulation and tumor cells to accurately release the free cytotoxic drugs, while non-cleavable linkers rely on enzymatic hydrolysis of the antibody component to release the payload complex. T-DM1 is a successful example of an ADC that uses a non-cleavable thioether linker. Enzyme-sensitive linkers, such as peptide-based linkers, can be cleaved for payload release in cells by specific enzymes often found in tumor regions.
Cytotoxic payload ADCs are a promising cancer treatment that involves attaching a potent cytotoxic payload to an antibody that targets cancer cells. The payload must be stable under physiological conditions and have a high potency, with an IC50 in the nanomolar or picomolar range. The most common types of payloads used in ADCs are tubulin inhibitors, DNA damaging agents, and immunomodulators. Tubulin inhibitors disrupt microtubule-dependent mitosis, while DNA damaging agents induce cell death through various mechanisms. Loncastuximab tesirine is the only ADC currently using PBD as the payload. ADCs with DNA damaging agents may work independently of cell cycles and may be effective against cells with low antigen expression.
Conjugation methods ADCs are commonly constructed using stochastic conjugation based on lysine and cysteine residues, but this method has some drawbacks such as unstable coupling and inconsistent DAR. To address these issues, several site-specific conjugation strategies have been developed, including introducing engineered reactive cysteine residues or unnatural amino acids with special functional groups. ThioMab technology is an example of the former approach, while the latter approach includes introducing N-acetyl-L-phenylalanine, azido methyl-L-phenylalanine, or azido lysine. These site-specific conjugation methods can produce ADCs with homogeneous DAR, high efficacy, good stability, and high safety, but each method has its own pros and cons. The choice of conjugation method depends on the specific characteristics of the ADC being constructed.
Mechanisms of ADCs for killing cancer cells
The image (Fig 9.3) depicts the various mechanisms by which ADCs can kill cancer cells. The upper-right panel illustrates the primary mechanism of action, which involves the binding of the ADC’s antibody component to the specific target antigen expressed on cancer cells. Once internalized by the cell, the cytotoxic payload is released, resulting in cell apoptosis or death via targeting DNA or microtubules. The antibody component of ADCs can bind to the antigen epitope of virus-infected or tumor cells, while the Fc segment binds to FCR on the surface of killer cells, mediating direct killing effects via ADCC, ADCP, and CDC. The antibody component of ADCs can also interfere with target function by inhibiting downstream signaling pathways, such as PI3K or MAPK, to induce cell apoptosis and inhibit tumor growth.
The Challenges and Potential Solutions in the Use of ADCs
Despite advancements in the development of new-generation ADCs with improved specificity and cytotoxicity profiles, there are still challenges that need to be addressed. One major challenge is the complex pharmacokinetic profiles of ADCs, which are influenced by factors such as target binding, elimination, and deconjugation. After administration, the concentration of both conjugated ADC and naked antibody decreases as the internalization of ADC and antibody clearance occurs. Antibody clearance is influenced by the mononuclear phagocyte system and FcRn-mediated recycling, which can prolong the half-life of antibodies, including conjugated ADC and naked antibodies. Free cytotoxic payload is mainly metabolized in the liver and excreted through urine or feces, which can be affected by drug interactions and damaged liver and kidney functions. Due to high interpatient variability, it is challenging to establish PK and PD models to describe the clinical characteristics of ADCs and assist in the design of new ADCs.
ADC Development Strategies in the Future
Mutant proteins are easier to internalize and degrade, making them ideal targets for ADC therapy. Bispecific ADCs targeting different sites on the same antigen can improve receptor aggregation and lead to rapid internalization of the target. Dual-payload ADCs using two distinct cytotoxic agents can reduce drug resistance and achieve more potent efficacy. New strategies, such as abandoning the traditional structure of mAb and using smaller molecular weight fragments, are being developed to improve penetration efficiency and payload delivery to tumor tissues. Payload selection is also evolving, with the use of more targeted drugs and immune drugs. Overall, there are still many innovative opportunities in ADC development.
Examples of ADCs in clinical use
There are currently several ADCs approved for clinical use, and they are listed below:
- Adcetris (brentuximab vedotin) – Hodgkin lymphoma and systemic anaplastic large cell lymphoma
- Kadcyla (ado-trastuzumab emtansine) – HER2-positive breast cancer
- Polivy (polatuzumab vedotin-piiq) – diffuse large B-cell lymphoma
- Enhertu (fam-trastuzumab deruxtecan-nxki) – HER2-positive breast cancer and metastatic non-small cell lung cancer
- Blenrep (belantamab mafodotin-blmf) – multiple myeloma
- Trodelvy (sacituzumab govitecan-hziy) – to treat triple-negative breast cancer and metastatic urothelial cancer.
Future Outlook for Precision Medicine in Oncology using ADCs
Antibody-drug conjugates (ADCs) are a type of precision medicine in oncology that uses monoclonal antibodies to selectively deliver a potent cytotoxic drug directly to cancer cells while sparing healthy cells.
The future outlook for ADCs in oncology is promising, as they offer several advantages over traditional chemotherapy drugs, including fewer side effects and improved efficacy. The use of ADCs is likely to continue to grow, with the development of new technologies and the increasing availability of biomarkers that can be used to identify patients who will benefit from treatment.
Advancements in ADC technology, such as the development of site-specific conjugation methods and the use of bispecific antibodies, are also expected to improve the therapeutic index and increase the range of cancers that can be targeted with ADCs. Additionally, new drug payloads are being developed that can overcome drug resistance and enhance the potency of ADCs.
Ongoing research and development in this field are likely to lead to the development of new and more effective ADCs that can be used to treat a wide range of cancers.
References
- Norsworthy, K. J. et al.,” FDA approval summary: mylotarg for treatment of patients with relapsed or refractory CD33‐positive acute myeloid leukemia”, Oncologist 23, 1103 (2018).
- Ethan Ennals for the Mail on Sunday. The new breed of drug which reduces bladder cancer deaths could replace chemotherapy in other cases. At daily mail. Co. UK (2021).
- Fu, Z., Li, S., Han, S., et al. Antibody-drug conjugates: the “biological missile” for targeted cancer therapy. Sig Transduct Target Ther 7, 93 (2022). https://doi.org/10.1038/s41392-022-00947-7.
- Lambert JM, Chari RVJ. Ado-trastuzumab emtansine (T-DM1): an antibody-drug conjugate (ADC) for HER2-positive breast cancer. J Med Chem. 2014; 57:6949–64.
- Dal Corso, A. et al. A non-internalizing antibody-drug conjugate based on ananthracycline payload displays potent therapeutic activity in vivo. J. Control. Release 264, 211–218 (2017).
- Miller DR. A tribute to Sidney Farber—the father of modern chemotherapy. Br J Haematol. 2006; 134:20–6.