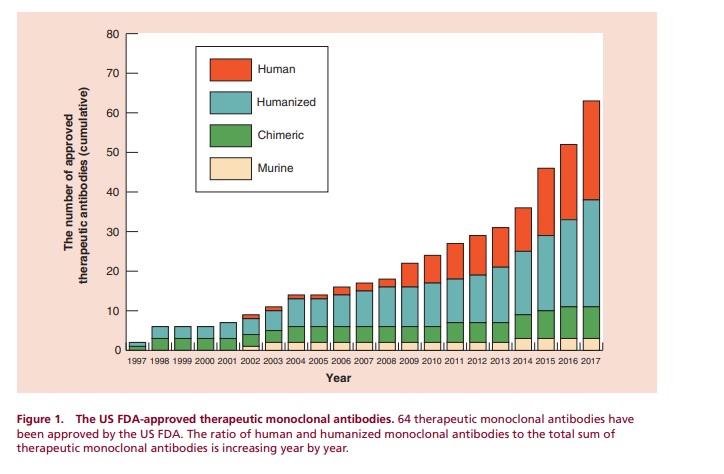
The biopharmaceutical market has been rapidly growing and is expected to continue expanding, with sales projected to reach over $380 billion by 2024. The number of approved therapeutic monoclonal antibodies has also increased significantly, with 64 approved by the US FDA in 2018 alone. The market is expected to continue to grow as efforts are made to explore new target diseases and improve formulations and dosage forms. Finding new target antigens will be crucial for the continued development of antibody-based medicines.
The number of therapeutic monoclonal antibodies approved by the US FDA has increased significantly since the late 1990s, with a total of 64 approvals in 2018. In 2017, 11 therapeutic monoclonal antibodies were approved, with a growing percentage of human and humanized antibodies. Next-generation products, such as bispecific antibodies, antibody-drug conjugates, sugar chain-modified antibodies, and low molecular weight antibodies, are gaining attention. Some of these products, including emicizumab, gemtuzumab ozogamicin, inotuzumab ozogamicin, and brentuximab vedotin, are already on the market.
Emerging Technologies in Monoclonal Antibody Development
Potential of bispecific antibodies for therapeutic use
Bispecific antibodies have been used for treating carcinomas, leukemia, and hemophilia as they can recognize two distinct antigens. Identifying suitable combinations of target molecules that can produce potent antibodies is crucial for developing new therapeutic bispecific antibodies. The EMARS method can analyze hetero-protein complexes formed on cell membranes under physiological conditions and identify cancer cell-specific bi-molecular complexes for preparing therapeutic bispecific antibodies against cancer cells. As there are infinite combinations of molecules, the EMARS method can help find suitable candidate antigens for developing new therapeutic approaches. Bispecific monoclonal antibodies with stereo-specific recognition can detect membranous antigens on cancer cells that are difficult to detect using conventional linear-specific monoclonal antibodies. They can also recognize unique conformational structures in heteroprotein complexes, suggesting that bi-molecular complexes can be detected as single molecular elements.
Potential Applications of Stereo-Specific Monoclonal Antibodies as Catalytic Agents
Recent research has shown that stereo-specific monoclonal antibodies could have potential applications as catalytic agents. Catalytic antibodies have unique characteristics that allow them to recognize and degrade antigens, making them superior to monoclonal antibody drugs. Studies have demonstrated the ability of catalytic antibodies to eradicate infections and reduce the accumulation of β-amyloid in mice. Stereo-specific monoclonal antibodies may provide more effective hydrolysis of intact antigens on cell membranes for new therapeutic medicine if the catalytic activity is introduced.
Antibody-drug conjugates
ADCs (antibody-drug conjugates) have a unique role in cancer treatment as they combine the specific targeting of monoclonal antibodies with the efficient killing effect of cytotoxic drugs. This precision targeting reduces off-target side effects and improves the therapeutic window. The mechanism of action involves the ADC binding to target antigens expressed on cancer cells and being endocytosed, eventually leading to the release of cytotoxic payloads in lysosomes, inducing cell apoptosis or death. The bystander effect of ADCs can enhance their efficacy by inducing cell death in neighboring cells. Additionally, ADCs can also mediate direct killing effects through ADCC, ADCP, and CDC effects. The antibody component of ADCs can inhibit downstream signal transduction pathways, inducing cell apoptosis. For example, trastuzumab of T-DM1 can bind to the HER2 receptor of cancer cells and inhibit signal transduction pathways to induce cell apoptosis.
Fc engineering
The regions of the Fc tail that are important for Fc interaction with FcγRs and C1q are the hinge and the proximal CH2 regions. These regions contain binding sites, and the CH2 domain’s structural conformation allows engagement with C1q or FcγR. Furthermore, the CH2 domains are modified post-translationally by glycosylation, which contributes to their stability and dynamics. Glycan components include core units of GlcNAc and mannose, with variations in galactose, bisecting GlcNAc, fucose, and sialic acid. Understanding these interactions has allowed for the modulation of C1q and FcγR binding through Fc engineering techniques such as site-directed mutagenesis, glycoengineering, and avidity modulation to enhance ADCC, ADCP, and CDC.
While hybridoma technology was groundbreaking for therapeutic antibodies, most approved mAbs today are produced using mammalian expression systems. These systems allow for higher yields and preservation of post-translational modifications, resulting in higher-quality mAbs. Variable regions from hybridoma or phage display technologies can be used in these systems. Chinese hamster ovary, mouse myeloma, and mouse hybridoma cell lines are commonly used, while human expression systems like HEK293 and CAP are available for preclinical purposes. Antibody sequence engineering at the Fc site can be achieved through site-directed mutagenesis or phage/yeast display libraries to find optimal Fc variants.
Next-generation sequencing and bioinformatics
Next-generation sequencing (NGS) can be used to sequence and analyze antibody repertoires to obtain a comprehensive understanding of their diversity and complexity. By amplifying and sequencing the variable regions of heavy and light chains, NGS can identify the frequency of each unique antibody sequence in a sample and provide information about the CDR3 length distribution, V (D) J gene usage, and somatic hyper mutation frequency. NGS can also be used to generate antibody libraries for further screening and optimization. By combining NGS with advanced computational algorithms, it is possible to identify and isolate highly specific and potent antibodies with desirable properties for various applications, such as diagnostics and therapeutics.
Monoclonal antibodies (mAbs) are artificially generated immunoglobulin with high binding affinity and are used extensively in various fields. However, the lack of proper classification and identification of mAbs has led to a reproducibility crisis. Confirming the amino acid sequence of antibodies is essential for improving research quality and understanding antibody-antigen interactions. While most approaches for antibody sequencing rely on DNA sequencing, they cannot detect important post-translational modifications. Therefore, protein-level sequencing is necessary, and tandem mass spectrometry (MS/MS) is a powerful method for this purpose. De novo peptide sequencing is commonly used to identify peptides from MS/MS spectra, and recent advances in deep learning have led to the development of various algorithms for peptide sequencing. These include DeepNovo, DeepNovo-DIA, SMSNet, PointNovo, and Casanovo, which have shown promising results for predicting peptide sequences from MS/MS data.
Novel Targets for Monoclonal Antibody Development
Immune checkpoint inhibitors
The discovery of immune checkpoints, specifically CTLA-4 and PD-1, has been a significant breakthrough in the development of cancer immunotherapy. These molecules were initially found to have a role in T cell activation or apoptosis, but further research demonstrated their crucial role in maintaining peripheral immune tolerance. Mice lacking these immune checkpoints developed autoimmune-like diseases that were either lethal in the case of CTLA-4 deficiency or appear later in life for PD-1 deficiency. In preclinical models, blocking CTLA-4 and PD-1 led to the development of effective anti-tumor immune responses, either as a single treatment or in combination with other therapies. The success of single-agent anti-CTLA-4 and anti-PD-1 therapies in humans was surprising given the previous preclinical research, but they have revolutionized cancer immunotherapy. Patients with metastatic melanoma, one of the most immunogenic human cancers, have experienced improved overall survival and long-term benefits from these treatments.
CAR-T cell therapy targets
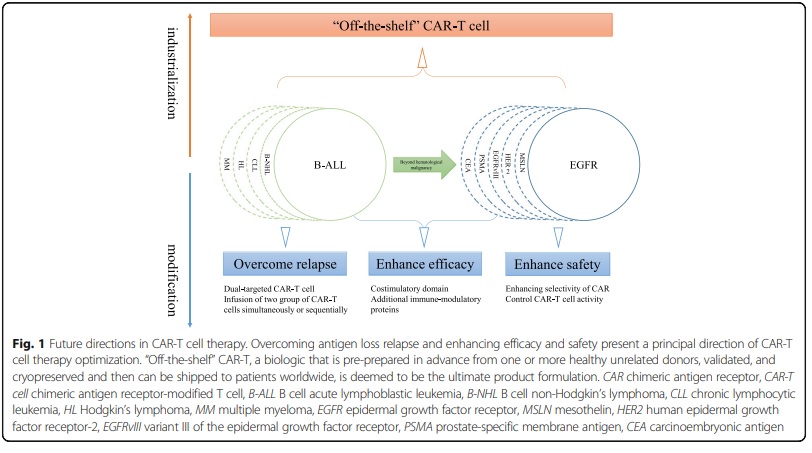
The chimeric antigen receptor (CAR) is a protein made up of different domains and is used to genetically modify T cells to recognize and attack specific cancer cells. Clinical trials using CAR-T cell therapy for B cell malignancies have shown promising results, especially in targeting CD19. However, the use of CAR-T cell therapy for solid tumors has faced challenges such as antigen loss relapse, on-target/off-tumor toxicity, less efficacy in solid tumors due to the tumor microenvironment, and difficulty in mass production. To overcome these challenges, researchers are exploring different strategies, including new CAR designs to improve safety and prevent tumor antigen escape relapse.
Novel cancer antigens
Recent progress in cancer immunotherapy has unveiled novel tumor-associated antigens (TAAs) that are specifically expressed in cancer cells and recognized as foreign by the immune system. These TAAs can originate from mutations, aberrant gene expression, or unique modifications. Immunotherapeutic strategies, such as cancer vaccines and adoptive T-cell therapy, have been developed to target these novel TAAs. Examples of novel TAAs include neoantigens, cancer-testis antigens, tumor-specific isoforms of proteins, and cancer stem cell antigens. Creating successful immunotherapy approaches is difficult because of the variety of cancer types and the ways in which tumors can avoid detection by the immune system.
Neurodegenerative disease targets
Neurodegenerative diseases are increasingly prevalent and are the seventh leading cause of death globally. They pose a significant challenge to researchers as they result in cognitive, motor, and other functional impairments due to the degeneration of neurons. These diseases are caused by the aggregation of proteins, namely amyloidal-β peptide, tau, α-synuclein, and mHTT, which result in altered neuronal function leading to cell death. Examples of such diseases include Alzheimer’s disease, Front temporal dementia, Corticobasal degeneration, Progressive supranuclear palsy, Parkinson’s disease, Multiple system atrophy, Dementia with Lewy-body, and Huntington’s disease. The available treatments can only alleviate symptoms and prolong life, but they have side effects and cannot cure the diseases. Although some clinical trials were discontinued due to severe side affects, a few of them showed positive effects in treating Alzheimer’s disease and Parkinson’s disease. Immunotherapeutic trials targeting amyloidal-β, tau, and α-synuclein have shown promising results, and preclinical studies targeting mHTT and prion proteins are currently being investigated. These clinical findings suggest that immunotherapy may have a promising role in treating neurodegenerative diseases.
References
- Akabane H. Issues of the biopharmaceutical industry and recommendations for further development. No. 71. Series of research papers (2018). www.jpma.or.jp/opir/research/rs 071/paper 71.pdf
- Hifumi E, Honjo E, Fujimoto N, Arakawa M, Nishizono A, Uda T., “Highly efficient method of preparing human catalytic antibody light chains and their biological characteristics. FASEB J. 26, 1607–1615 (2012)”.
- Staudacher, A. H. & Brown, M. P., “Antibody-drug conjugates and bystander killing: is antigen-dependent internalization required?” Br. J. Cancer 117, 1736–1742 (2017).
- Van der Horst HJ, Nijhof IS, Mutis T, Chamuleau MED, “Fc-Engineered Antibodies with Enhanced Fc-Effectors Function for the Treatment of B-Cell Malignancies. Cancers” (Basel). 2020 Oct 19; 12(10):3041. Doi 10.3390/cancers12103041. PMID: 33086644; PMCID: PMC7603375.
- Choi, H. L., Yang, H. R., Shin, H. G., Hwang, K., Kim, J. W., Lee, J. H., & Lee, S. (2023). Generation and Next-Generation Sequencing-Based Characterization of a Large Human Combinatorial Antibody Library. International Journal of Molecular Sciences, 24(6), 6011.
- Mondal A.C. (2023), “Immunotherapeutic Approaches for the Treatment of Neurodegenerative Diseases: Challenges and Outcomes. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders)”.



