The quality problems in medical testing labs were brought to the attention of Congress through media stories, leading to the enactment of the Clinical Laboratory Improvement Amendments of 1988 (CLIA ’88). The aim of CLIA ’88 was to address concerns over unreliable lab test results that could harm public health by establishing national standards and regulations for quality control. These regulations were enforced through periodic inspections to ensure compliance. The need for minimum qualifications for technical personnel, supervisors, and lab directors were recognized, although there was little empirical evidence to support this decision at the time of enactment. Overall, CLIA ’88 sought to prevent misdiagnosis caused by inaccurate lab results.
The Clinical Laboratory Improvement Amendments (CLIA) of 1988 provides federal regulatory standards for clinical laboratory testing to ensure the accuracy and reliability of laboratory tests. CLIA requires laboratories to obtain certification, undergo proficiency testing, and adhere to quality control standards. Laboratories are also required to follow guidelines for personnel qualifications, facilities, and equipment, as well as to maintain accurate records and provide patients with accurate and understandable test results. The certification process includes an inspection of the laboratory’s facilities, equipment, and procedures. Proficiency testing requires laboratories to test specimens provided by an external agency and report their results for evaluation. Laboratories must meet performance standards to maintain certification. These measures ensure that laboratory tests are accurate, reliable, and consistent, and help to protect the public’s health.
How CLIA ensures the accuracy and reliability of laboratory tests?
The establishment of CLIA was justified due to the crucial role laboratory testing plays in providing effective healthcare, and the importance of accurate and reliable testing for maintaining public health in the US. Unregulated laboratories may offer cheaper testing, but with higher risks of inaccuracy and unreliability, giving them an unfair advantage over-regulated laboratories. This can undermine the effectiveness of health and safety regulations and existing standards are inadequate for protecting public health and welfare, as well as eliminating burdens on interstate commerce.
Regulatory Standards for Laboratory-Developed Tests (LDTs) and Quality Management Systems
Currently, laboratory-developed tests (LDTs) do not need to be approved by the FDA before they are sold, as long as they are developed, produced, and used within a single laboratory that meets the requirements of the Clinical Laboratory Improvement Amendments (CLIA) certification. False or incorrect test results can pose a significant risk to individuals and lead to misinformation, particularly when testing quality is inadequate. Thus, laboratories must establish a comprehensive quality management system that includes sample handling, processing, storage, test method validation, equipment upkeep, staff training, and other quality measures that ensure precise test results. Laboratories utilizing human biospecimens must adhere to relevant quality standards and conduct tests within a quality management system that undergoes external review for compliance. By implementing such measures, research labs can help generate dependable and reproducible research outcomes, in keeping with CLIA regulations and standards for accuracy.
Proposed Changes to HbA1c Testing Regulations
The Centers for Medicare & Medicaid Services and Centers for Disease Control and Prevention proposed a rule change in February 2019 to update proficiency testing regulations for clinical laboratories, including HbA1c. The acceptable performance criterion for HbA1c measurement was set at ±10%. However, the Clinical Laboratory Improvement Amendments of 1988 are proposing changes to proficiency testing regulations based on biological variability between patients. Critics argue that the proposed acceptance limit of ±10% for HbA1c would compromise patient safety and accuracy in managing diabetes mellitus.
Accuracy and Standardization of HbA1c Assays for Diabetes Diagnosis and Management
HbA1c tests are accurate with a proficiency limit of 6% and don’t need to be relaxed to 10%. Standardization and improvements have been made by NGSP, CAP, and manufacturers, with tighter acceptance limits in proficiency testing. These efforts have enhanced HbA1c measurements, and it’s recommended for diabetes diagnosis. Relaxing the criteria could lead to misdiagnosis or missed diagnosis of diabetes. Over the past 27 years, the acceptable limit for HbA1c proficiency testing has decreased since the foundation of CLIA in 1992. HbA1c has vital roles in diabetes diagnosis, management, and drug approval. CLIA’s proposal to increase the acceptable limit from ±6% to ±10% would decrease the effectiveness of HbA1c assays and put patients’ safety at risk.
Importance of QC Procedures and Specific Metrics for Next-Generation Sequencing (NGS) Assays
QC procedures are crucial in monitoring assay components, such as reagents, specimen processing, instrumentation, and data processing. The workgroup focused on QC metrics specific to NGS for accurate results during patient sample testing. Metrics include coverage depth and uniformity, quality scores for alignment and base calling, allelic read percentage, strand bias, GC bias, and signal intensity decline.

Evaluating the Quality, Accuracy, and Reliability of Laboratory Tests Based on CLIA Requirements
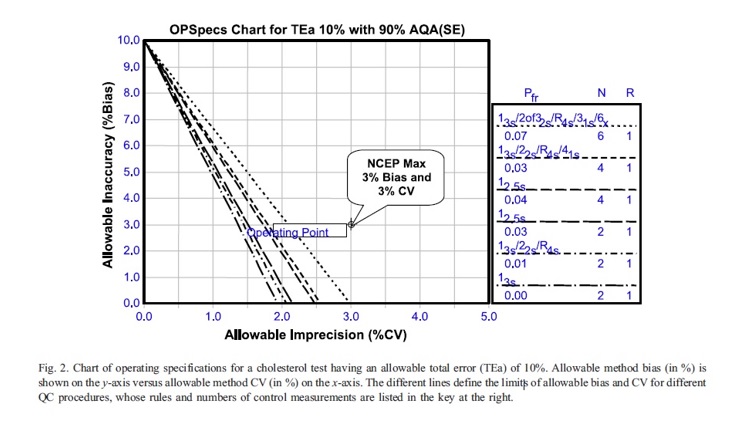
The quality of a laboratory test can be evaluated based on two types of requirements: analytical quality, which can be allocated over the analytical components of precision, accuracy, and quality control, and clinical quality, which considers pre-analytical factors such as the patient’s biological variation. Quality-planning models can be used to allocate these requirements mathematically, and OP Specs (Operation specification chart) charts and power function graphs can be used to visually represent the relationship between precision, accuracy, and QC necessary for the reliable detection of medically important errors. The OP Specs chart for a cholesterol test shows that a method’s operating point is given by its observed bias and SD/CV, and a method with a maximum allowable CV and bias of 3% cannot meet the quality required for proficiency testing.
CLIA as a Model for Regulating Forensic Science
Establishing CLIA was based on the following findings: Laboratory testing is crucial for providing effective healthcare. Accurate and reliable testing is essential for protecting public health, laboratory testing often involves interstate commerce or has a significant impact on it, unregulated laboratories can offer cheaper testing but with a higher risk of inaccuracy and unreliability, giving them an unfair advantage over-regulated laboratories. Unregulated laboratories can undermine the effectiveness of health and safety regulations that apply to other laboratories, existing regulatory standards and procedures do not adequately protect public health and welfare, nor do they eliminate burdens on interstate commerce, and federal regulation is a reasonable and appropriate means of promoting public health and welfare while protecting interstate commerce.
To improve the quality and reliability of forensic science, the criminal justice system should learn from the experiences of the medical community and adopt CLIA as a framework for regulating forensic science on a national level. It is the responsibility of forensic science practitioners, the criminal justice system, and lawmakers at the state and federal levels to adopt CLIA to create a system for improving forensic laboratories. This system could be called the forensic laboratory improvement amendments (FLIA) and would address the specific needs of the criminal justice system.
References
- US Department of Health and Human Services. Clinical laboratory improvement amendments of 1988 (CLIA) proficiency testing regulations related to analytes and acceptable performance 2019. https://www.govinfo.gov/content /pkg/FR-2019-02-04/pdf/2018-28363.pdf. Accessed March 18, 2019.
- Westgard QC. Quality requirements: desirable biological variation database specifications 2019. https://www.westgard.com/biodatabase1.htm. Accessed March 18, 2019.
- Clinical and Laboratory Standards Institute (CLSI).Quality management system: a model for laboratory services; approved guideline 4th ed. (Document no. GP26–A4, Vol. 31, No. 15) (Clinical and Laboratory Standards Institute, 2011).
- Centers for Medicare and Medicaid Services. Clinical laboratory improvement amendments of 1988 (Part 493) 1443–1495 (US Department of Health and Human Services, 1988).
- Westgard JO. Charts of operational process specifications (”OPSpecs Charts”) for assessing the precision, accuracy, and quality control needed to satisfy proficiency testing criteria Clin Chem 1992; 38:1226–33.
- Parkinson DR, McCormack RT, Keating SM, Gutman SI, Hamilton SR, Mansfield EA, et al. Evidence of clinical utility: an unmet need in molecular diagnostics for patients with cancer. Clin Cancer Res 2014; 20:1428–44.
- Donnell Christian (2011) How the Clinical Laboratory Improvement Amendments (CLIA) Can Improve Forensic Laboratory Quality, Forensic Science Policy & Management: An International Journal, 2:1, 18-27, DOI: 10.1080/19409044.2010.549926.



