Genome sequencing involves decoding the order of the four chemical building blocks, or nucleotides, that make up an individual’s DNA. This process provides a wealth of information about an individual’s genetic makeup, including variations and mutations that may be associated with the disease.
Personalized medicine is a new approach to medical practice that utilizes an individual’s genetic makeup (Genomic sequences) to make informed decisions about disease prevention, diagnosis, and treatment. Genetic information can assist doctors in selecting the most appropriate medication or therapy, as well as determining the correct dosage or treatment plan. The Human Genome Project’s findings have been instrumental in advancing personalized medicine.
Personalized medicine was originally proposed in the field of genetics, in the sense that by the application of nano genomics and nano proteomics, it is possible to tailor medical interventions to the specific molecular picture of the individual.
Personalized medicine signifies a change in strategy from universal treatment to individualized care and customized therapies. The integration of advanced technologies, such as genomics, improves our comprehension of diseases and their molecular mechanisms, leading to more personalized interventions. In addition, other types of diagnostic tests, such as transcriptomics (tests based on RNA), metabolomics (analysis of metabolites in an individual), and proteomics (analysis of all expressed proteins), are becoming increasingly significant.
The advancement of personalized medicine in many ways is being driven by the intersection of big data analytics and WES. The figure below illustrates the changing paradigms of personalized medicine. Notable timelines in Genomics and Personalized Medicine are showcased, including the data storage size of the four big data domains by 2025, with genomics either on par or the most demanding of the domains. However, there are many barriers still for WES to having wider use in mainstream medical practice. The major challenges include results reproducibility, reporting standards, and affordability.
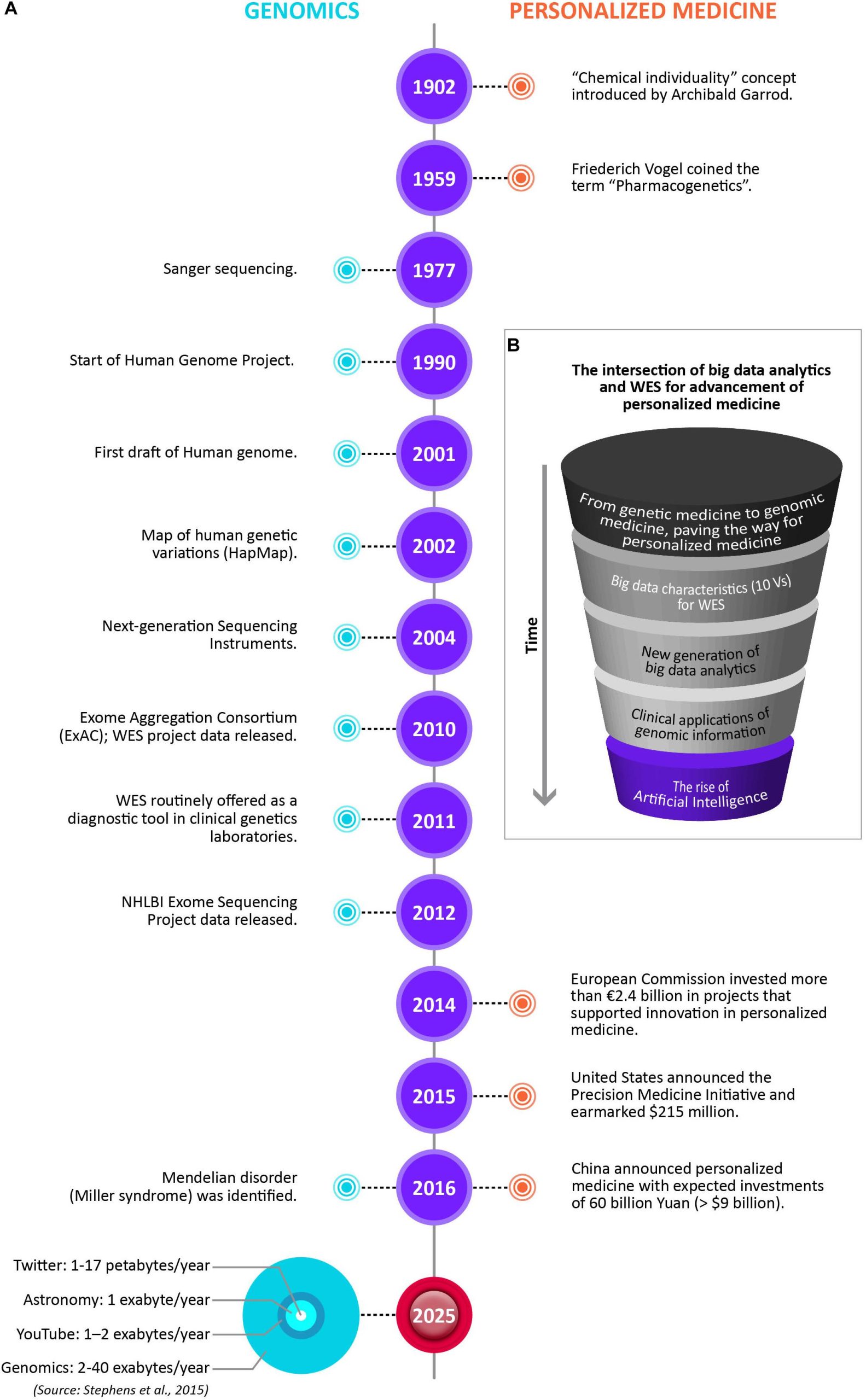
The Role of genome sequencing in personalized medicine
The advancements in cfDNA analysis for prenatal testing have led to the possibility of obtaining whole-genome sequencing of a growing fetus. In the future, it may also be possible to obtain whole-genome sequencing of a patient’s tumor at an early stage of recurrence, using a non-invasive method for surveillance that could facilitate early intervention. However, to ensure that these surveillance strategies are effective, tests with high sensitivity and specificity need to be established through large randomized control trials. This is necessary to avoid the potential harm caused by false positive or ambiguous results. In the long run, personalized sequencing may become an essential aspect of comprehensive cancer care, including risk assessment, prevention, disease screening and diagnosis, personalized pharmacogenomic-based therapy, and post-therapy surveillance.
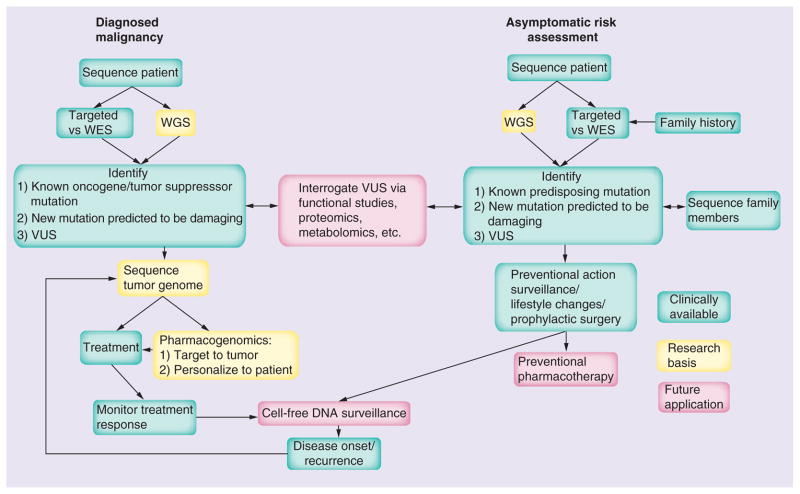
The degree to which genomic information is utilized in medical practice is closely tied to the progress made in genomic technologies and sciences. The limited clinical application and slow initial adoption of medical genomics can be attributed to the insufficient clinical evidence supporting its use in multifactorial diseases. As a result, there has been a greater emphasis on variants that have a high or nearly certain correlation probability between genotype and phenotype (known as high penetrance) in the early stages.
Methods of Genome Sequencing Used in Personalized or Precision Medicine
The several methods of genome sequencing that are used in personalized medicine, including:
- Whole Genome Sequencing (WGS) involves sequencing an individual’s entire genome, including both coding and non-coding regions. This method provides the most comprehensive information about an individual’s genetic makeup and is useful in identifying disease-causing mutations and variants.
- Whole Exome Sequencing (WES) focuses on sequencing only the exome, which is part of the genome that codes for proteins. This method is less expensive and faster than WGS, but provides less comprehensive information about an individual’s genome.
- Targeted Sequencing focuses on specific regions of the genome that are associated with a particular disease or condition. This method is useful in identifying disease-causing mutations and variants in specific genes.
- RNA Sequencing is used to analyze the transcriptome, which is the set of all RNA molecules in a cell. This method provides information about gene expression and can be used to identify changes in gene expression associated with a particular disease or condition.
- Single-Cell Sequencing: Single-cell sequencing is used to analyze the genome of individual cells, providing information about genetic variation and gene expression at the cellular level. This method is useful in identifying cell types and subtypes, and in studying complex biological systems such as cancer.
These methods of genome sequencing are becoming increasingly accessible and affordable, allowing for greater use in personalized medicine. Each method has its strengths and limitations, and the choice of method depends on the specific research question or clinical application.
Genomic Data and Personalized Medicine
Adverse drug reactions (ADRs) are reported to be one of the major causes of morbidity and mortality that can easily be avoided. In the United States, 3% of registered drugs carry FDA recommendations for genetic tests. Some reports suggest that polygenic risk scores for complex medical conditions can achieve a level of predictive power that is comparable to genetic risk assessments for monogenic diseases.
Personalized medicine is mostly focused on pharmacological interventions that are tailored to an individual’s ability to respond favorably to treatment. This response is determined by the person’s metabolic capability to process the medication. The CYP450 family of enzymes is responsible for the initial phase of metabolizing foreign substances, and genetic variants within these enzymes can alter their activity. Identifying these genetic variants can help predict how a drug will be processed by the body, which can assist doctors in selecting treatments that will be effective and safe, without causing toxicity.
Applications of Genome Sequencing in Personalized Medicine
NGS has not only improved our understanding of cancer but has also given birth to a new way of treating cancer patients called Precision Medicine (PM). This approach tailors medical therapy to the unique characteristics of each individual and their condition, including the genomic alterations present in their cancer. While not a new concept, the use of NGS and the availability of large-scale human genome databases have created an opportunity for significant progress in this area.
We have already moved away from a “one-size-fits-all” approach to medicine and are using a more progressive stratification of patients based on their disease subtype, clinical features, and biomarkers (Stratified Medicine). NGS has the potential to lead the shift towards Precision Medicine, where a wide range of patient features and the mutational scenario of their cancer can be taken into account to select the best therapeutic approach in oncological care.
Challenges of Genome Sequencing
Genetic variations stand to impact significantly on the risk and survival outcome of a patient’s health. The Precision Medicine Initiative raises ethical, social, and legal issues related to privacy and informed consent. It is expensive, and developing drugs for specific genetic variations are likely to be costly. Also, reimbursement from third-party payers may also be an issue. Healthcare providers will need to learn more about molecular genetics and biochemistry to incorporate precision medicine into routine healthcare.
Ethical Considerations
Precision medicine using genome sequencing raises several ethical considerations, including:
Informed Consent: Patients must be fully informed about the risks and benefits of genome sequencing and provide informed consent before their genetic data can be collected and analyzed. It is important to ensure that patients understand the implications of genetic information and are not coerced into undergoing sequencing.
Privacy and Confidentiality: Genomic data is highly sensitive and personal information. Patients must be assured that their data will be kept confidential and used only for approved purposes. Researchers and healthcare providers must have secure systems in place to protect genomic data from breaches or unauthorized access.
Discrimination: Genetic information can be used to discriminate against individuals in areas such as employment, insurance, and education. To prevent discrimination, there should be policies in place to ensure that genetic information is not used against individuals.
Stigmatization: Genetic testing can reveal information that could stigmatize individuals or their families, such as the risk of developing certain diseases. It is important to minimize the potential for stigmatization and ensure that patients receive appropriate counseling and support.
Equity: The availability of genomic testing and precision medicine may not be equal across different populations, leading to disparities in healthcare access and outcomes. Efforts should be made to ensure that precision medicine is accessible and affordable for all patients, regardless of their socioeconomic status or geographic location.
Commercialization: As precision medicine becomes more common, there is a risk of commercialization and the prioritization of profit over patient welfare. Regulations should be in place to ensure that genomic testing and precision medicine are used ethically and in the best interests of patients.
Future Directions
The use of genome sequencing has the capability to pave the way for personalized medicine in various potential avenues in the future.
- Early detection and prevention of diseases can be achieved by identifying genetic variants through genome sequencing
- Targeted therapies can be developed based on genomic data, leading to more effective and safer treatments
- Pharmacogenomics can help select the most appropriate drug and dose for an individual patient by identifying genetic variations that affect drug metabolism or efficacy
- Gene editing technologies such as CRISPR-Cas9 can be used to treat genetic diseases by correcting or removing disease-causing mutations
- Integrating genomic data with other sources of health information can provide a complete picture of a patient’s health, leading to more informed treatment decisions and personalized care plans
- Genome sequencing can also be used to study the genetic basis of diseases on a population level, leading to more effective public health interventions and policies.
The advancement of personalized medicine in many ways is being driven by the intersection of big data analytics and WES.
Conclusion
In conclusion, genome sequencing has a significant and growing role in precision medicine. It has the potential to transform the way we diagnose, treat, and prevent diseases by providing personalized information about an individual’s genetic makeup. As technology continues to improve, the cost of sequencing is decreasing, and the amount of genomic data available is increasing. This is leading to the development of new tools and techniques that will enable healthcare providers to use genomic data to tailor treatments to individual patients. While there are still ethical, social, and legal issues that need to be addressed, the future of precision medicine looks promising with genome sequencing at its core.
References
- Eisen, M. B et.al; (1998), “Cluster analysis and display of genome-wide expression patterns”, Proceedings of the National Academy of Sciences of the United States of America, 95(25), 14863–14868.
- NHS England Personalised medicine. www.england.nhs.uk/ourwork/qual-clin-lead/personalisedmedicine/ [Accessed 15 September 2017]
- “Advancing Personalized Medicine Through the Application of Whole Exome Sequencing and Big DataAnalytics”, Pawel Suwinski et.al; published: 12 February 2019, doi: 10.3389/fgene.2019.00049
- Edward D Esplin et.al, “Personalized sequencing and the future of medicine: discovery, diagnosis, and defeat of disease”, Pharmacogenomics. 2014 Nov; 15(14): 1771–1790, doi: 10.2217/pgs.14.117
- Lobo, I. (2006). Multifactorial inheritance and genetic disease. Nat. Educ. 1:5. Google Scholar
- FDA (2018). Science & Research (Drugs) – Table of Pharmacogenomic Biomarkers in Drug Labeling. Silver Spring, MD: FDA. [Google Scholar]
- Khera A. V., Chaffin M., Aragam K. G., Haas M. E., Roselli C., Choi S. H., et al. (2018). Genome-wide polygenic scores for common diseases identify individuals with risk equivalent to monogenic mutations. Nat. Genet. 50 1219–1224. 10.1038/s41588-018-0183-z [PMC free article] [PubMed]
- Evans W. E., Relling M. V. (2004). Moving towards individualized medicine with pharmacogenomics. Nature 429 464–468. 10.1038/nature02626.
- Shin, S. H., Bode, A. M., & Dong, Z. (2017). Precision medicine: the foundation of future cancer therapeutics. NPJ Precision Oncology, 1(1), 12
- Dawood, S., Broglio, K., Gonzalez-Angulo, A. M., Buzdar, A. U., Hortobagyi, G. N., and Giordano, S. H. (2008). Trends in survival over the past two decades among white and black patients with newly diagnosed stage IV breast cancer. J Clin. Oncol. 26, 4891–4898. doi: 10.1200/JCO.2007.14.1168
- Stephens Z. D., Lee S. Y., Faghri F., Campbell R. H., Zhai C., Efron M. J., et al. (2015). Big Data: astronomical or Genomical? PLoS Biol. 13:e1002195. 10.1371/journal.pbio.1002195 [PMC free article]



