Clinical laboratory testing in the US is regulated by both Clinical Laboratory Improvement Amendments (CLIA) and the FDA. FDA clearance is required for diagnostic tests that are commercially marketed as kits and operate in low to moderate-complexity laboratories. However, the FDA approval process is time-consuming and expensive, which limits the types of tests and sample types that can be submitted for approval. CLIA oversees all aspects of laboratory operations, including personnel requirements, quality programs, and validation requirements for laboratory-developed tests (LDPs). Accreditation can be achieved through deemed agencies or state-specific regulations. The New York State Department of Health has stricter regulations for patient protection, particularly for molecular oncology testing.
CLIA compliance is integrated into clinical laboratory operations, including training, proficiency testing, and unannounced inspections. Laboratories must perform a validation process and document the results for any tests not reviewed by the FDA, such as LDPs. Peer reviews of the validation data are conducted by CMS or organizations with deemed status overseeing laboratories, like CAP. Molecular pathology has specific criteria for peer inspections. Laboratories must also participate in proficiency testing twice a year; sharing and assessing inter-laboratory data and reporting results for unknown samples. Results are graded and compared to other laboratories.
Evolution of Clinical Laboratory Standards
The need for clinical laboratory standards was identified in the late 1960s when cytology laboratories faced issues with overworked personnel and high error rates in reading PAP smears. In 1967, the Clinical Laboratory Improvement Amendment was introduced to establish the first set of regulations for laboratory standards, mainly for independent and hospital laboratories. Later, in response to an article published in The Wall Street Journal in November 1987 highlighting the issue of false negative pap smears and subsequent claims, the Clinical Laboratory Improvement Act of 1988 (CLIA 88) was passed. The primary goal of CLIA 88 was to comprehensively regulate gynecologic cytology laboratories.
Ensuring Standards and Certificates for Clinical Laboratory Testing
The CLIA Program, established under the Clinical Laboratory Improvement Amendment, provides standards and certificates for clinical laboratory testing in facilities that test human specimens for diagnostic, preventive, or therapeutic purposes, and for health assessments. It aims to ensure accurate and timely test results, regardless of where the test is conducted. Most Laboratories developed Tests are regulated under this program, but the FDA has started discussions on regulating some LDTs (Laboratory Developed Tests). Each laboratory system, assay, and examination is graded for complexity, based on seven criteria, with scores ranging from 1 to 3. The CLIA Program is primarily operated by the Centers for Medicare and Medicaid Services (CMS), funded by user fees collected from approximately 200,000 laboratories in the United States.
The criteria include knowledge, training, and experience of laboratory staff, preparation of reagents and materials, operational steps and their characteristics, calibration, quality control, proficiency testing materials, test system troubleshooting and equipment maintenance, and interpretation and judgment of test results. A score of 1 to 3 is assigned for each criterion, with 1 being the lowest level of complexity and 3 indicating the highest level. Score 2 is assigned for tests with characteristics intermediate between those for scores of 1 and 3.
Why CLIA was established?
According to CLIA, tests and test systems that meet specific requirements for risk, error, and complexity are given a CLIA certificate of waiver. The US Congress revised the CLIA waiver provisions in November 2007 to clarify that tests approved by the FDA for home use are automatically eligible for CLIA waiver. However, a significant number of waived tests are not performed according to proper protocols, with more than 50% being performed incorrectly, resulting in medical errors, some of which can be fatal.
Importance and Classification of Clinical Laboratories in Healthcare
Clinical laboratories are essential healthcare facilities that aid in the diagnosis, treatment, and management of patients. They are staffed by trained medical technologists who perform various tests on biological specimens from patients. Clinical laboratories can be government-owned or privately owned, and they can be general or specialty laboratories. Specialty laboratories can provide disease-specific diagnostic and confirmatory tests in fields like clinical chemistry, clinical microbiology, hematology, and molecular biology. They can also provide tests for public health, water analysis, and environmental substances.
Clinical laboratories can be classified according to their level of operation, as peripheral, intermediate-level, or national reference laboratories. Laboratory networks have been developed to foster proper coordination and collaboration among clinical laboratories. Ensuring quality in clinical laboratories is crucial, and this involves addressing various significant issues such as standardizing laboratory services, strengthening laboratory systems, and creating new and efficient diagnostic tools. Clinical laboratories follow a strict three-phase laboratory testing process: pre-analytical, analytical, and post-analytical. Despite the advanced technology in modern-day clinical laboratories, the results still depend heavily on the accuracy and reliability of laboratory professionals.
How does (CLIA) classify the complexity level of laboratory tests performed in physician office laboratories?
According to the CLIA regulations, clinical laboratories are certified at the highest level of test complexity, and the Centers for Medicare and Medicaid Services (CMS) oversee these regulations. Other organizations can also be granted authority to carry out inspections and offer accreditation. There are three categories of testing under CLIA regulations: waived, provider-performed microscopy (PPM), moderate complexity, and high complexity. Non-waived testing refers to the three categories other than waived. A physician group previously performed only waived testing, which is the lowest complexity under CLIA, such as finger stick glucose, pregnancy kits, urine dipsticks, and some urine drug abuse screening strips. The physician group has added clinical chemistry and hematology testing, which fall under moderate complexity. PPM, a subgroup of moderate complexity, includes microscope examination of urine sediment by physicians or other providers, potassium hydroxide preparations, pinworms preparations, Fern tests, nasal smears for granulocytes, fecal leukocyte examinations, and qualitative semen analysis (limited to presence or absence of sperm and the detection of motility).
Regulation of Clinical Laboratory
The FDA and CMS regulate medical devices and clinical laboratories, respectively. Tests are categorized by complexity, with waived tests being the simplest and non-waived tests being either moderate or high complexity. FDA-cleared tests that run on automated analyzers are typically classified as moderately complex. High complexity testing includes any test categorized by the FDA as high risk and all LDTs, which CLIA defines as tests developed, manufactured, and used within a single laboratory or modified FDA-approved assays. Laboratories must extensively validate and establish performance specifications for LDTs before implementing them clinically. Laboratories regulated by NYSDOH must submit LDT validation, calculated measures of diagnostic accuracy, standard operating procedure manuals, and quality assurance methods to the NYSDOH. After a risk assessment and application review, tests may be approved for patient use.
Timeline of Clinical Laboratory Regulation
Laboratories that perform moderate and/or high complexity testing must have a Certificate of Accreditation and undergo biennial inspections from authorized organizations such as the College of American Pathologists (CAP) or the Joint Commission (TJC). These inspections ensure compliance with the CLIA standards for test performance, quality monitoring, and personnel requirements. High-complexity testing labs must also meet additional training, education, and certification requirements. While the FDA claims regulatory authority over all in-vitro diagnostic devices (IVDs), it has chosen not to regulate laboratory-developed tests (LDTs) under CLIA. In 2014, the FDA proposed that labs performing LDTs comply with the same FDA process as manufacturers seeking IVD approval, but this was met with opposition from stakeholders. The FDA withdrew the proposal in 2016 and has since been working on multiple drafts for LDT oversight with Congress and stakeholders.
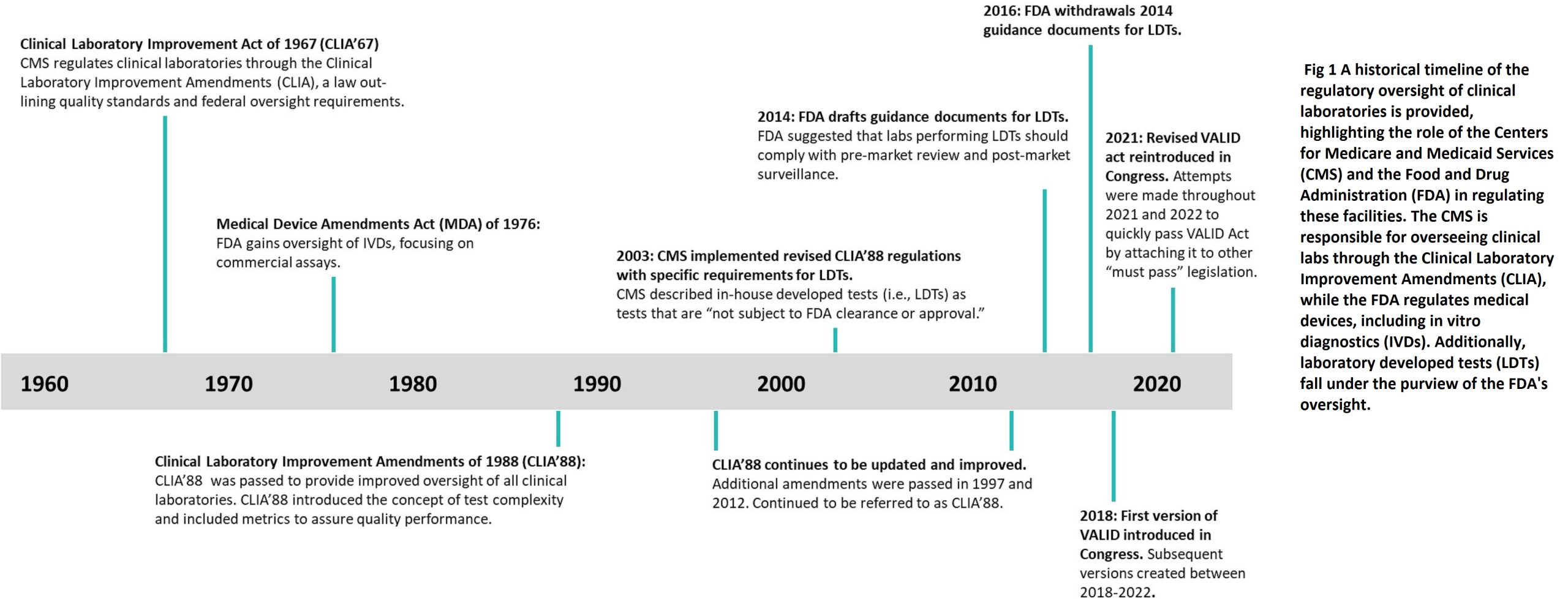
The VALID Act
The VALID Act was first introduced in 2018 and has since undergone revisions to address concerns from the laboratory medicine community. The 2021 version proposed a risk-based framework for in vitro clinical tests (IVCTs), including both commercial IVDs and in-house LDTs. High-risk tests would require pre-market review, with an option for technology certification to reduce the review process’s stringency. Low-risk tests would be exempt from pre-market review. The act offered a grandfathering provision for existing LDTs if certain criteria were met. However, the act’s approach was flawed as it overlooked the fact that the clinical laboratory is already a highly regulated environment. LDTs undergo rigorous validation before implementation and are continuously monitored through quality control checks, proficiency testing, and inspections. Laboratories participating in quality review programs must submit their validations for approval.
- Potential Implications of the VALID Act
If the FDA were to regulate LDTs, patient care would be negatively impacted due to the inability of smaller labs to afford FDA approval, leading to a decrease in the number of LDTs offered. As a result, patient samples would need to be sent to larger reference labs, causing delays in care. In the case of measuring immunosuppressants, a quick turnaround time is necessary to adjust the patient’s dosage accurately. Immunoassay methods may offer faster results, but they are subject to interference from metabolites and similar drugs. The VALID Act failed to consider the successful oversight of clinical laboratories by CLIA, CAP, and state agencies, and instead of granting the FDA oversight, updating CLIA standards and integrating reforms into the current infrastructure is a more viable alternative.
A Compliance CLIA certificate is given to a laboratory that has passed an inspection verifying that it meets all the necessary CLIA requirements. This certificate permits the laboratory to conduct moderate or high complexity testing or both, as well as waived tests.
Importance of Maintaining CLIA Compliance in Clinical Laboratories
Maintaining CLIA compliance is crucial for clinical laboratories for several reasons, including:
- Ensuring patient safety: CLIA regulations are designed to ensure that clinical laboratories operate safely and accurately to produce reliable test results. By complying with these regulations, laboratories can reduce the risk of errors that could cause harm to patients.
- Meeting regulatory requirements: Compliance with CLIA regulations is required by law for all clinical laboratories that perform testing on human specimens. Failure to comply with these regulations can result in penalties, fines, and even suspension of laboratory operations.
- Maintaining accreditation: Compliance with CLIA regulations is a key factor in obtaining and maintaining accreditation from organizations such as the College of American Pathologists (CAP) and The Joint Commission (TJC). Accreditation assures patients, healthcare providers, and regulatory agencies that the laboratory meets high standards for quality and safety.
- Ensuring quality of test results: CLIA regulations require clinical laboratories to participate in proficiency testing programs and implement quality control procedures to ensure the accuracy and reliability of test results.
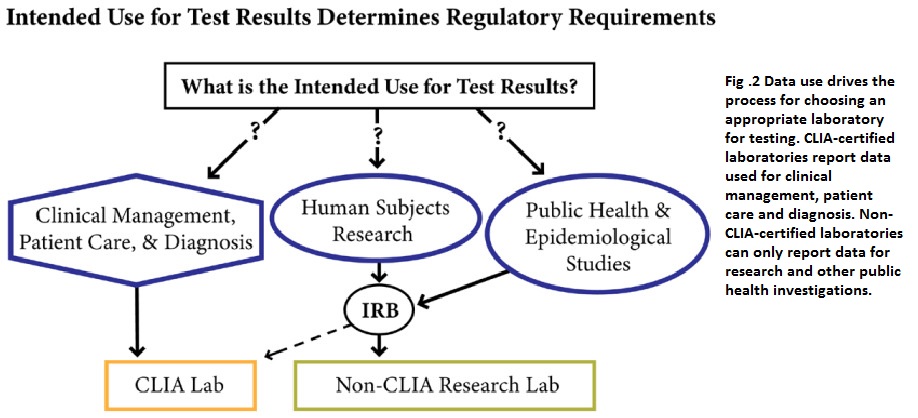
When selecting a laboratory for testing, the data required determines whether a CLIA-certified or non-CLIA-certified laboratory is appropriate. CLIA-certified laboratories can report data for clinical management, patient care, and diagnosis, while non-CLIA-certified laboratories can only report data for research and public health investigations.
Call to Action for Laboratories to Prioritize CLIA Compliance
Clinical laboratories must prioritize CLIA compliance to ensure patient safety, meet regulatory requirements, maintain accreditation, and provide accurate and reliable test results. Non-compliance with CLIA regulations can result in penalties, fines, and suspension of laboratory operations, which can have significant consequences for patient care. Laboratories can prioritize CLIA compliance by regularly reviewing and staying up-to-date on CLIA regulations, conducting internal audits to identify non-compliance areas and taking corrective action, participating in proficiency testing programs, implementing quality control procedures, and providing regular training for staff on CLIA regulations and quality control procedures. Prioritizing CLIA compliance will improve the quality and safety of laboratory services, maintain accreditation, and provide better patient care.
References
- “Clinical Laboratory Improvement Act (CLIA)”. New Mexico Department of Health. Retrieved”, 2023-04-18.
- Anderson, Richard E. (May 2005). , “Medical Malpractice – A Physician’s Sourcebook”. Totowa, New Jersey: Humana Press, pp. 168–169. ISBN 1-58829-389-0.
- Kotlarz VR. Tracing our roots: “origins of clinical laboratory science”, Clin Lab Sci. 1998 Jan-Feb; 11(1):5-7.
- Simundic AM, Lippi G., “Pre analytical phase–a continuous challenge for laboratory professionals. Biochem Med (Zagreb). 2012; 22(2):145-9.
- R. Genzen, et.al, “Laboratory-developed tests: a legislative and regulatory review”, Chem., 63 (2017), pp. 1575-1584, 10.1373/clinchem.2017.275164
- J. Soldin, B.W. Steele, D.L. Witte, E. Wang, R.J. Elin, Lack of specificity of cyclosporine immunoassays: results of a college of American Pathologists Study, Arch. Pathol. Lab. Med. 127 (2003) 19–22, https://doi.org/10.5858/2003-127-19-LOSOC.



