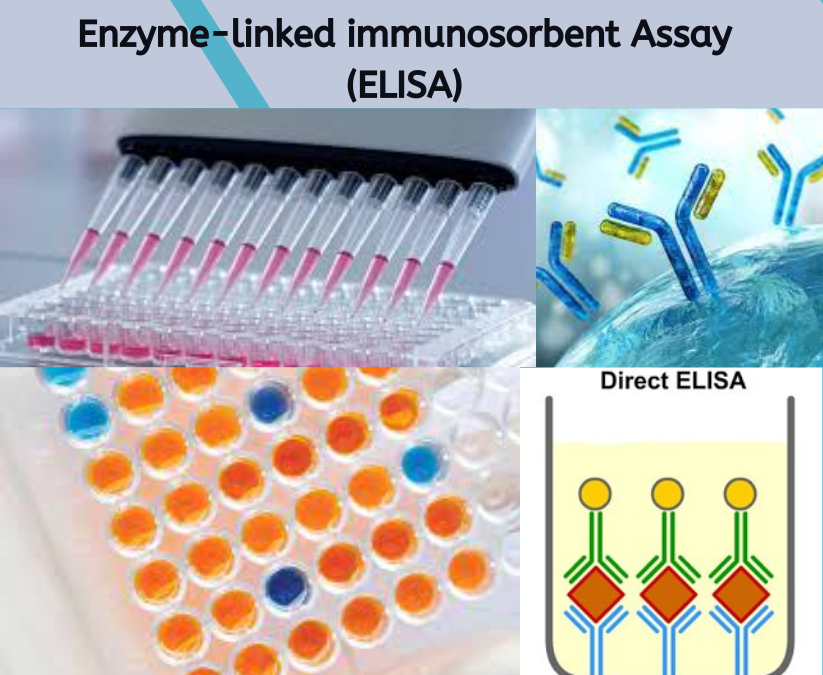
The enzyme-linked immunosorbent assay (ELISA) is a highly sensitive and widely used laboratory technique for the detection and quantification of various analytes, such as proteins, antibodies, hormones, drugs and food allergens. This test is based on the principle of specific antigen-antibody interactions, where an antibody-specific to the target epitopes binds selectively to the analyte. ELISA is versatile and can be adapted to detect a wide range of substances in different sample matrices, including blood and other biological fluids.
Developed in the 1970s, ELISA leverages the specific interaction between an antigen and an antibody, along with enzymes like horseradish peroxidase (HRP) or alkaline phosphatase, to generate a detectable signal. This signal is often visualized on plates with coated surfaces that capture the antibody-specific to the target antigen. The activity of the conjugate enzyme, linked to the detection antibody, is measured by the color change resulting from the enzymatic reaction with the substrate, producing quantifiable values that indicate the presence and amount of the target analyte.
ELISA is used in various immunoassays due to its adaptability in components and formats. For example, the sandwich ELISA format is particularly effective for detecting large molecules, where the analyte is captured between two antibodies. In contrast, competition assays are used when detecting small molecules or when the antigen is too small to be captured by two antibodies.
The integration of ELISA with other techniques, like Western blot, provides detailed information about protein expression and modification, which are important in studying diseases and biological processes. ELISA is also essential in clinical diagnosis, as it aids in identifying disease markers, pathogens, and antibodies in patient samples. By optimizing the material used for the assay and ensuring minimal possibility of cross-reactivity, ELISA can produce reliable results in both research and diagnostic settings. References to validated methods and protocols further support its widespread use and application in diverse fields.
Elisa Procedure:
1. Coating:
The ELISA protocol begins with coating a microtiter plate with a coating solution containing an antigen or capture antibody. This step immobilizes the antigen or antibody onto the plate surface, creating a solid-phase immunoassay. The choice of antigen or capture antibody depends on the specific target analyte and application, such as detecting cytokines, viruses, or hormones. Proper adsorption and immobilization are crucial for assay sensitivity and specificity. Factors such as the concentration of the coating solution and the type of plate used play an important role in the effectiveness of this step. The surface of the plate must provide optimal binding sites for the capture molecule to ensure high specificity and minimal non-specific binding.
2. Blocking:
To prevent non-specific binding, a blocking step is performed by adding a blocking solution like bovine serum albumin (BSA) or casein. This step reduces background signal and enhances the assay’s reliability. The blocking agent occupies any uncoated sites on the plate, minimizing unwanted interactions during subsequent steps. Proper blocking is crucial to maintaining the stability of the assay and ensuring accurate results, as it prevents the binding of non-target molecules, which could otherwise affect the outcome. For example, biotin and streptavidin are commonly used in some ELISA formats to facilitate strong, specific binding of antibodies or antigens to the plate surface.
3. Sample and Standard Addition:
After blocking, samples and standards are added to the plate. Standards, which are known concentrations of the target analyte, are used to create a calibration curve, while unknown samples, such as serum or plasma, are tested in parallel. Accurate pipetting and proper dilution of samples in the appropriate buffer are vital for generating reliable data. The type of sample and the matrix in which the analyte is present can influence the assay’s outcome, necessitating careful selection and preparation of reagents and controls. Each sample’s specific characteristics must be considered to avoid interference in the detection of the target molecules.
4. Primary Antibody Incubation:
The primary antibody, which is specific to the target antigen, is added to the wells. This antibody binds to the antigen, forming an antigen-antibody complex. The primary antibody can be either directly labeled with an enzyme or, in sandwich ELISAs, detected by a secondary enzyme-conjugated antibody. The epitope of the antigen recognized by the primary antibody is crucial, as it determines the specificity and sensitivity of the detection. Incubation is typically performed at room temperature to ensure optimal binding. In cases where highly specific detection is needed, antibodies conjugated with biotin can be used, followed by detection with streptavidin-enzyme conjugates to enhance signal strength.
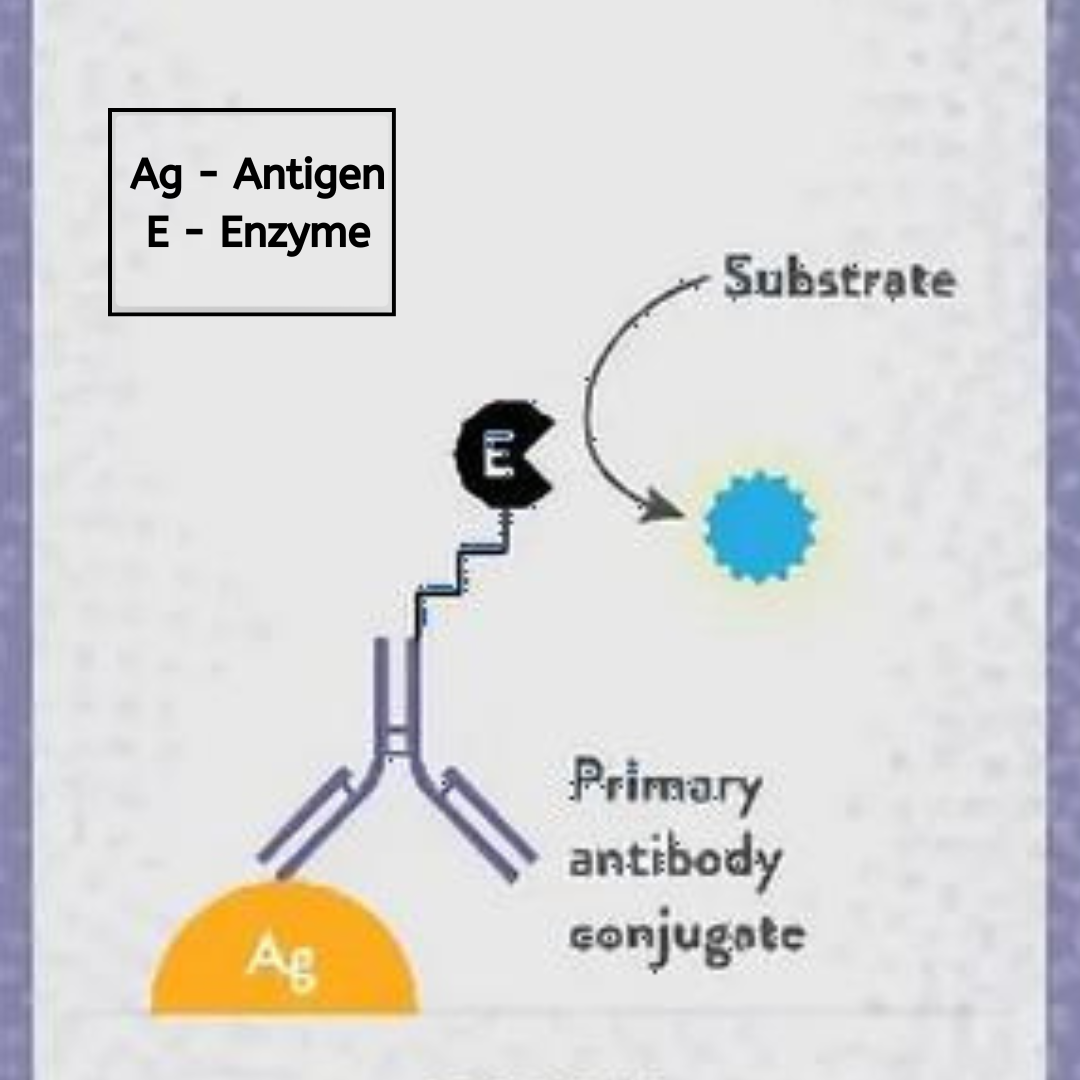
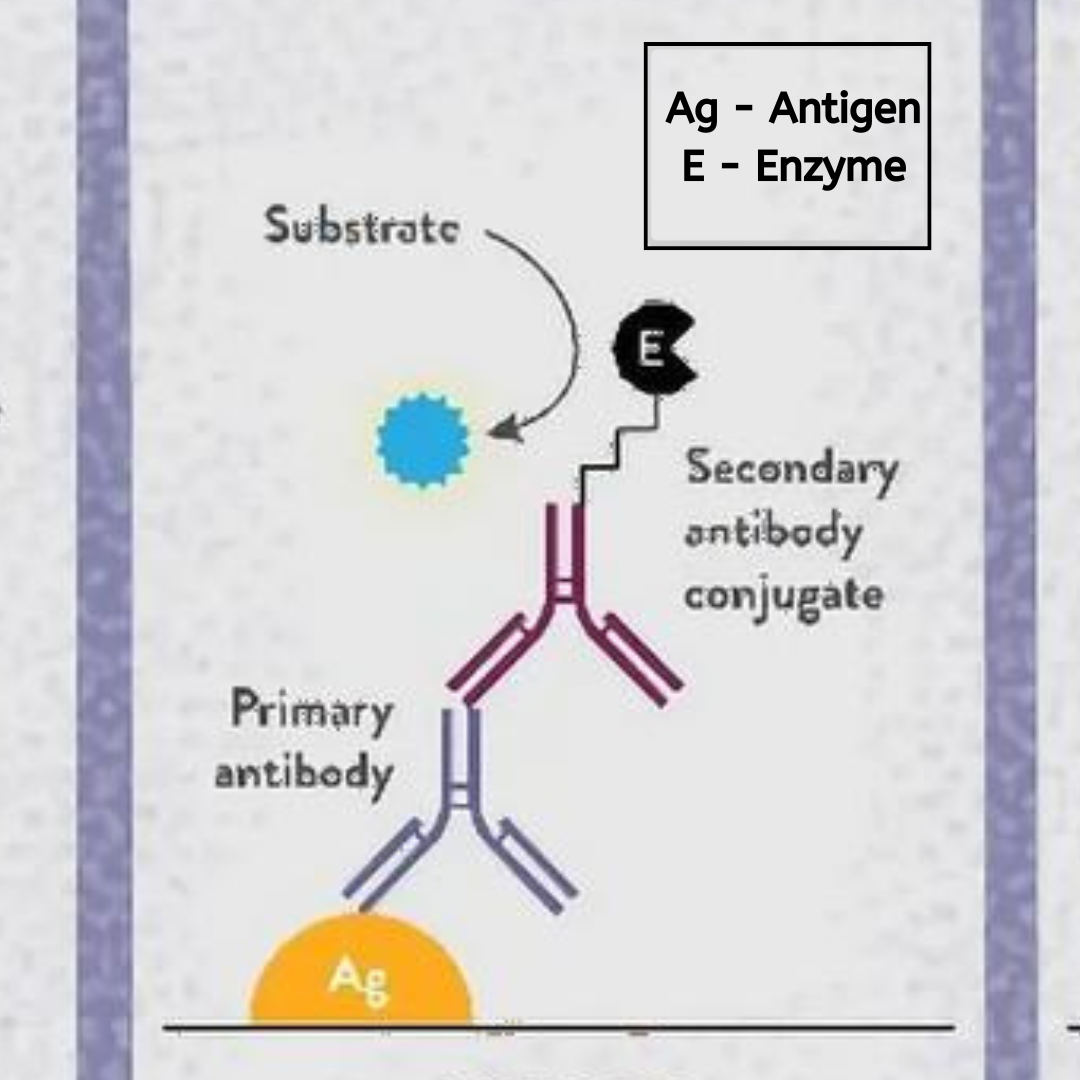
5. Secondary Antibody Incubation:
If the primary antibody is not directly labeled, a secondary antibody conjugated to an enzyme like HRP or alkaline phosphatase is added. This step amplifies the signal and enhances detection sensitivity. The enzyme-conjugated antibody binds to the primary antibody, completing the sandwich complex in the case of sandwich ELISAs. Proper washing steps are essential after each incubation to remove unbound reagents and reduce background noise, ensuring a clear signal from the bound antigen-antibody complexes. This step may also involve competitive binding assays where a known amount of labeled antigen competes with the sample antigen for binding to the capture antibody, providing quantitative information about the sample’s analyte concentration.
6. Substrate Addition:
A substrate solution specific to the enzyme is added to each well. For HRP, the substrate might be TMB (3,3′,5,5′-tetramethylbenzidine), which produces a color change upon reaction. The intensity of the color is directly proportional to the amount of antigen present and can be measured using a spectrophotometer or plate reader. This step’s timing, substrate concentration, and incubation conditions are critical for optimal signal generation. The selection of substrates and the conditions under which they react are fundamental in the overall assay performance. Substrates must be chosen based on their stability and the sensitivity required for the detection of specific amounts of the target antigen.
7. Stop Solution:
A stop solution, such as sulfuric acid, is added to halt the enzymatic reaction. This ensures that the signal remains stable for measurement and analysis. The final color intensity, measured at a specific absorbance wavelength, reflects the concentration of the target analyte in the sample. Proper handling of the stop solution and maintaining consistent timing across wells are important to ensure uniform results. The use of stop solutions also helps stabilize the signals, ensuring that the color development does not continue and that the data reflects a true endpoint for quantification.
Considerations and Advantages
Its ability to use different types of assays, such as direct, indirect, sandwich, and competitive formats, adds to its versatility. This flexibility allows for various configurations and applications depending on the target analyte, sample type, and desired outcome.
However, ELISA also has some disadvantages, such as potential cross-reactivity, variations in results due to inconsistent reagent quality or protocol adherence, and the need for proper control and validation. The method requires careful optimization of each step, including reagent selection, washing conditions, incubation times, and storage conditions, to ensure accurate and reproducible results. Factors like the quality of the detection antibody and the stability of the reagents used can greatly impact the reliability of the results. Competitive assays, for example, must be meticulously controlled to ensure accurate measurements of antigen concentration in the presence of other molecules.
Experiment and Instrumentation
Instrumentation used in ELISA experiments is essential for obtaining accurate and reliable results. The use of automated plate washers and dispensers, incubators, and microplate readers ensures consistency in the execution of ELISA protocols. Automation minimizes human error and allows for high-throughput screening, which is particularly advantageous in large-scale studies or when testing numerous samples. For optimal performance, instrumentation must be properly calibrated and maintained according to manufacturer specifications, and all protocols should be rigorously followed to maintain the integrity of the experimental data.