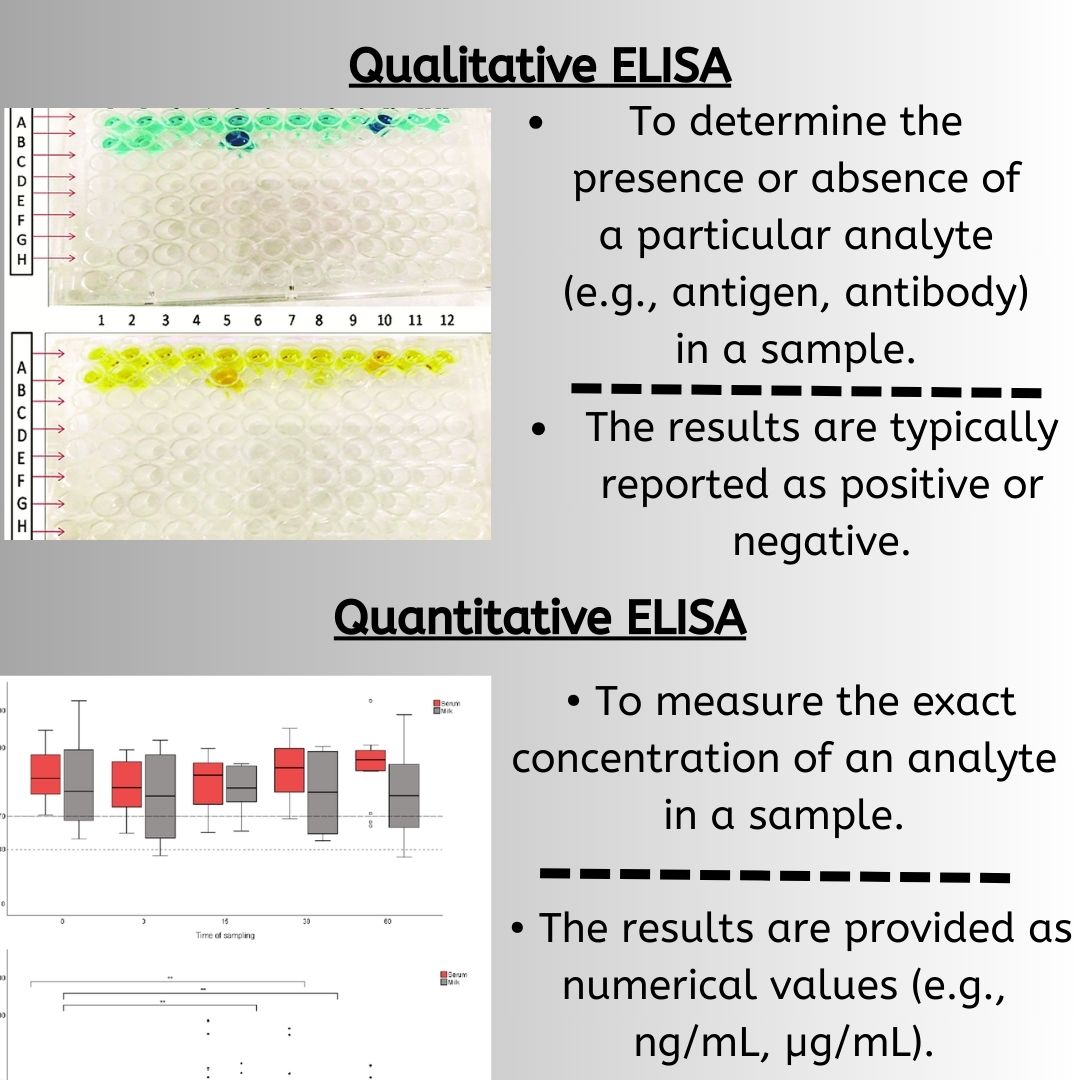
Enzyme-Linked Immunosorbent Assay (ELISA) is a solid-phase immunosorbent technique widely used for the detection and quantification of specific proteins, antibodies, or antigens in a sample. The different types of ELISA are tailored for various applications and offer unique advantages in terms of sensitivity, specificity, and versatility. In all these methods, the choice of reagents, such as the enzyme and substrate for detection, plays a crucial role in determining the sensitivity and specificity of the assay. Common detection methods include colorimetric, fluorescence, and chemiluminescence, each offering different advantages depending on the application.
Direct ELISA:
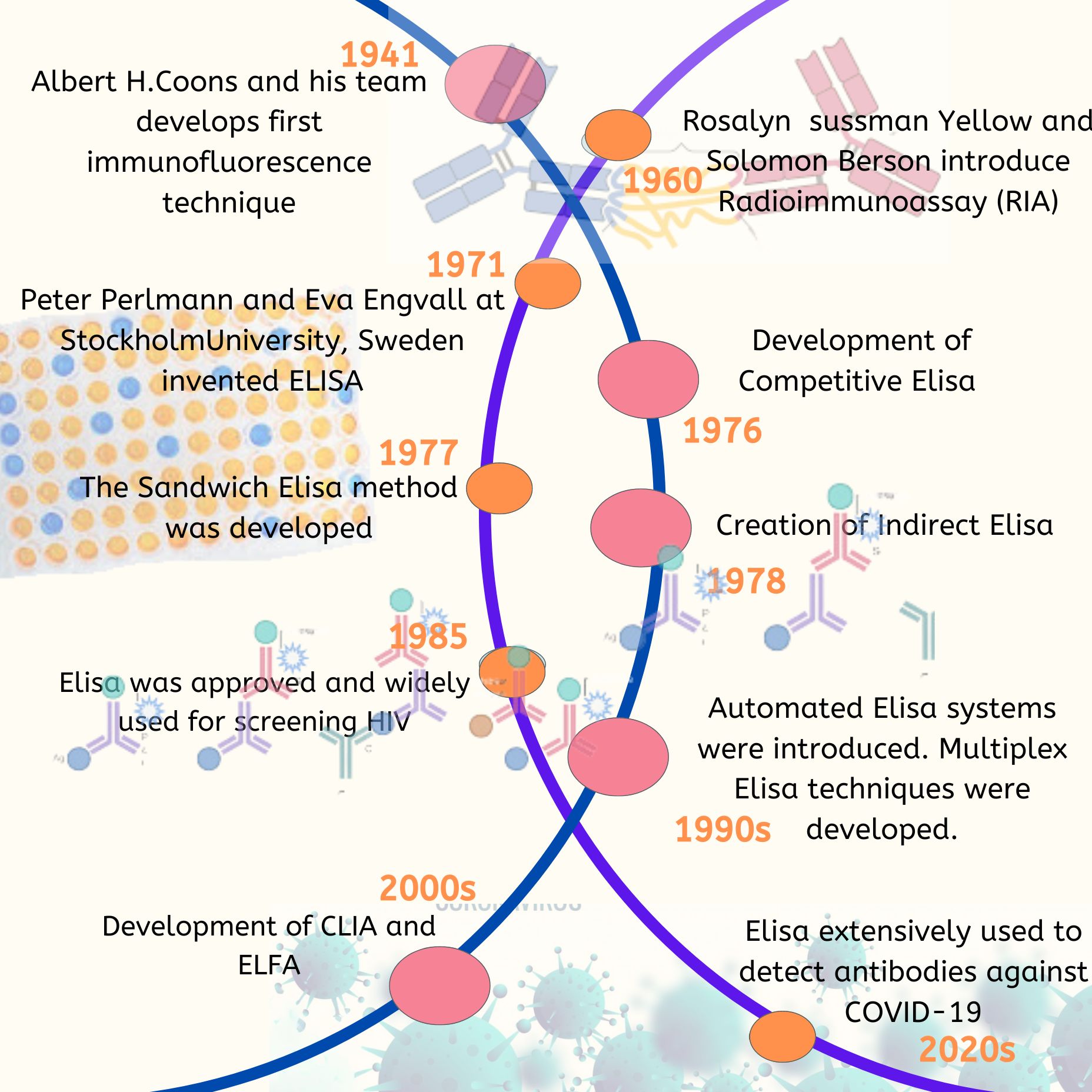
- Engvall, Perlmann, Van Weemen, and Schuurs independently developed direct ELISA in 1971, paving the way for other ELISA types.
- This method effectively detects large antigens. The plate is coated with the antibody or antigen directly, and an enzyme-linked antibody or antigen is used for detection. It is often used for quantitative analysis, offering quick and straightforward results. However, it may have lower sensitivity due to minimal amplification, and inhibition effects can be more pronounced.
- After incubation, any unbound antigens or antibodies are washed away. A substrate is added to create a visible signal, usually a color change. The intensity of this signal is measured to determine the amount of antigen or antibody present.

|
Advantages: |
|
|
Disadvantages: |
|
Indirect ELISA:
- Lindström and Wager developed the indirect ELISA technique in 1978, inspired by direct ELISA, to measure porcine IgG. Indirect ELISA is key in clinical diagnostics. It detects antibodies in blood, showing past exposure to diseases such as Lyme disease, HIV, and bird flu.
- With indirect ELISA, a secondary antibody is used to detect and separate the antigen instead of the primary antibody.
- During incubation, if antibodies against the antigen are present in the serum, antigen-antibody complexes form. To visualize these complexes, a secondary antibody tagged with an enzyme that binds specifically to the primary antibody is added. Indirect ELISA is widely used in endocrinology to find antigens.
- This method enhances sensitivity and can be used for both qualitative and quantitative assays.

|
Advantages: |
|
|
Disadvantages: |
|
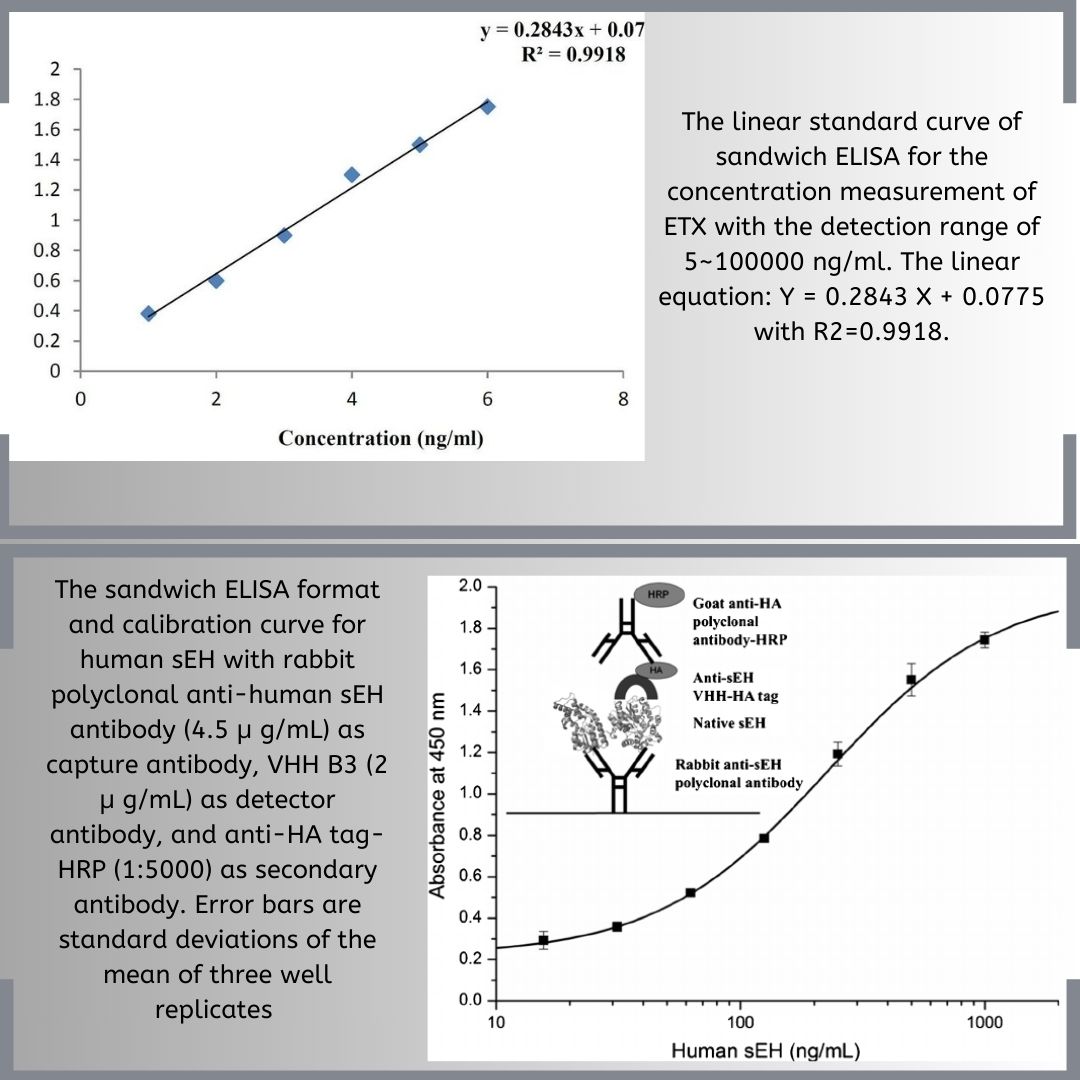
Sandwich ELISA:
- Kato and his team developed this technique in 1977. Sandwich ELISA identifies different strains of pathogens and is useful when the pathogen or antigen is limited.
- Microplate wells are coated with a capture antibody and blocked to prevent non-specific binding. The sample containing the antigen is then added.
- The plate is incubated so the antigens can bind to the capture antibodies. After incubation, a wash removes unbound antigens, leaving only the specific ones attached.
- This method is particularly useful for quantitative analysis in complex samples. Detection can be achieved using various reagents that produce colorimetric, fluorescence, or chemiluminescence signals.
- Coloration indicates a positive result, showing enzyme activity and antigen presence. No color means a negative result, indicating no antigen.
- The target antigen is “sandwiched” between two antibodies, giving the method its name, “Sandwich ELISA.” This method is 2-5 times more sensitive than other types of ELISA.

|
Advantages: |
|
|
Disadvantages: |
|
Competitive ELISA:
- Yorde and his team developed this method in 1976. Competitive assays are used when the antigen being measured is small and has only one binding site for antibodies.Wells are coated with either an antigen-specific antibody or an antibody-specific antigen. The sample and an enzyme-tagged antigen or antibody are added together.
- The tagged and untagged molecules compete to bind to the wells. After washing away unbound molecules, an enzyme substrate is added, causing a color change. It can be used for both qualitative and quantitative analysis, with detection often relying on colorimetric, fluorescence or chemiluminescence signals.
- The color intensity is inversely related to the analyte concentration: low levels of the antigen or antibody result in high absorbance, while high levels result in low absorbance.

|
Advantages: |
|
|
Disadvantages: |
|
Multiplex ELISA:
Multiplex immunoassays are powerful for studying changes in diseases, helping with monitoring and treatment improvements. Multiplex ELISA expands upon the sandwich ELISA by detecting multiple epitopes on antigens or samples within a single microtiter plate. This advancement resembles protein array formats, enabling simultaneous detection of numerous antigens in one well.

|
Advantages: |
|
|
Disadvantages: |
|
References
-
- Suleyman Aydin, A short history, principles, and types of ELISA, and our laboratory experience with peptide/protein analyses using ELISA, Peptides, Volume 72, 2015, Pages 4-15, ISSN 0196-9781
- Long Wu, Guanghui Li, Xin Xu, Lin Zhu, Riming Huang, Xiaoqiang Chen, Application of Nano-ELISA in food analysis: Recent advances and challenges, TrAC Trends in Analytical Chemistry, Volume 113, 2019, Pages 140-156, ISSN 0165-9936
- Verma, J., Saxena, S., & Babu, S. G. (2012). ELISA-Based Identification and Detection of Microbes. Analyzing Microbes, 169–186. doi:10.1007/978-3-642-34410-7_13 10.1007/978-3-642-34410-7_13
- Tighe, P. J., Ryder, R. R., Todd, I., & Fairclough, L. C. (2015). ELISA in the multiplex era: potentials and pitfalls. PROTEOMICS–Clinical Applications, 9(3-4), 406-422.
- Okda, F., Liu, X., Singrey, A., Clement, T., Nelson, J., Christopher-Hennings, J., … & Lawson, S. (2015). Development of an indirect ELISA, blocking ELISA, fluorescent microsphere immunoassay, and fluorescent focus neutralization assay for serologic evaluation of exposure to North American strains of Porcine Epidemic Diarrhea Virus. BMC Veterinary Research, 11, 1-14.
- Shafie, M. H., Antony Dass, M., Ahmad Shaberi, H. S., & Zafarina, Z. (2023). Screening and confirmation tests for SARS-CoV-2: benefits and drawbacks. Beni-Suef University journal of basic and applied sciences, 12(1), 6.